Sandbox myosinkinesin
From Proteopedia
| Line 2: | Line 2: | ||
<StructureSection load='4uxr' size='340' side='right' caption='This is the head region of kinesin' scene=''> | <StructureSection load='4uxr' size='340' side='right' caption='This is the head region of kinesin' scene=''> | ||
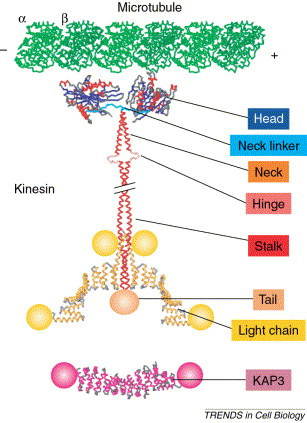
| + | [[Image:1-s2.0-S0962892402024005-gr1.jpg]] | ||
| + | This is all the parts of a Kinesin Dimer | ||
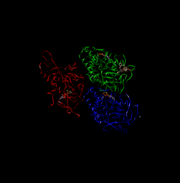
| - | + | [[Image:4UXR.png | thumb | This is 4UXR, the head of kinesin.]]<ref name= "BLAHHH">doi:10.1016/S0962-8924(02)02400-5</ref> | |
| - | [[Image:4UXR.png | thumb | This is 4UXR, the head of kinesin.]] | + | |
== Function == | == Function == | ||
| - | Kinesin is generally responsible for the transport of polypeptides and other large molecules throughout a single cell, such as moving the spindles during cell division. Kinesin can only move from the negative (-) end of microtubules to the positive (+) end. This means that kinesin only moves objects out towards the periphery of the cell and thus aids in the secretion of molecules or division of cells.<ref>doi: 10.2210/rcsb_pdb/mom_2005_4</ref> | + | Kinesin is generally responsible for the transport of polypeptides and other large molecules throughout a single cell, such as moving the spindles during cell division. Kinesin can only move from the negative (-) end of microtubules to the positive (+) end. This means that kinesin only moves objects out towards the periphery of the cell and thus aids in the secretion of molecules or division of cells.<ref>doi: 10.2210/rcsb_pdb/mom_2005_4</ref> By binding to ATP this causes a conformational change that allows the head group to bind to the microtubule, which causes a pivot in the neck region and eventually drags the entire protein along the microtubule. <ref name= "NECK"/><ref name= "OP"/> |
| + | |||
| + | == Diseases == | ||
| + | Research is still being done, but possible medical problems associated with kinesin failure could include mitotic/meiotic diseases (Down syndrome, etc.), failure of cilia properly working could influence organs like the kidney (polycystic kidney disease), and neuropathy (Alzheimers, etc.). | ||
| + | <ref name= “BLAHHH” / > | ||
| + | |||
| + | |||
== Structural highlights == | == Structural highlights == | ||
Revision as of 18:35, 17 December 2015
Kinesin
| |||||||||||
References
- ↑ Mandelkow E, Mandelkow EM. Kinesin motors and disease. Trends Cell Biol. 2002 Dec;12(12):585-91. doi: 10.1016/s0962-8924(02)02400-5. PMID:12495847 doi:http://dx.doi.org/10.1016/s0962-8924(02)02400-5
- ↑ doi: https://dx.doi.org/10.2210/rcsb_pdb/mom_2005_4
- ↑ 3.0 3.1 3.2 Rice S, Lin AW, Safer D, Hart CL, Naber N, Carragher BO, Cain SM, Pechatnikova E, Wilson-Kubalek EM, Whittaker M, Pate E, Cooke R, Taylor EW, Milligan RA, Vale RD. A structural change in the kinesin motor protein that drives motility. Nature. 1999 Dec 16;402(6763):778-84. PMID:10617199 doi:http://dx.doi.org/10.1038/45483
- ↑ 4.0 4.1 4.2 4.3 4.4 Song YH, Marx A, Muller J, Woehlke G, Schliwa M, Krebs A, Hoenger A, Mandelkow E. Structure of a fast kinesin: implications for ATPase mechanism and interactions with microtubules. EMBO J. 2001 Nov 15;20(22):6213-25. PMID:11707393 doi:10.1093/emboj/20.22.6213
- ↑
Structural highlights
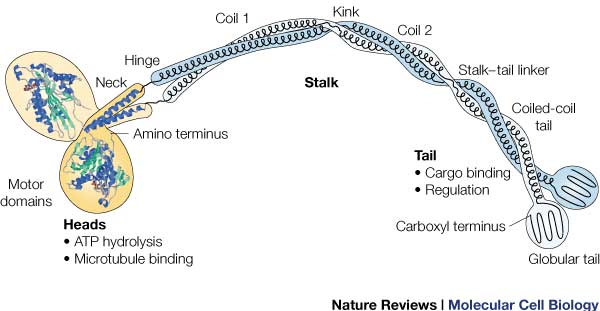
<li id="cite_note-RAS-7">↑ <sup>[[#cite_ref-RAS_7-0|8.0]]</sup> <sup>[[#cite_ref-RAS_7-1|8.1]]</sup> Kull FJ, Sablin EP, Lau R, Fletterick RJ, Vale RD. Crystal structure of the kinesin motor domain reveals a structural similarity to myosin. Nature. 1996 Apr 11;380(6574):550-5. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/8606779 8606779] doi:[http://dx.doi.org/10.1038/380550a0 http://dx.doi.org/10.1038/380550a0]</li></ol></ref> <ref>Hackney, David. "Kinesin-1 Structure." Duke. Duke, 25 Jan. 2005. Web. 17 Dec. 2015. <https://labs.cellbio.duke.edu/kinesin/KinesinStructure.html>.</li>
<li id="cite_note-5">[[#cite_ref-5|↑]] Rayment I, Rypniewski WR, Schmidt-Base K, Smith R, Tomchick DR, Benning MM, Winkelmann DA, Wesenberg G, Holden HM. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993 Jul 2;261(5117):50-8. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/8316857 8316857]</li>
<li id="cite_note-6">[[#cite_ref-6|↑]] Krukau A, Knecht V, Lipowsky R. Allosteric control of kinesin's motor domain by tubulin: a molecular dynamics study. Phys Chem Chem Phys. 2014 Apr 7;16(13):6189-98. doi: 10.1039/c3cp53367k. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/24561904 24561904] doi:[http://dx.doi.org/10.1039/c3cp53367k http://dx.doi.org/10.1039/c3cp53367k]</li>
<ref>Hackney, David. "Kinesin-1 Structure." Duke. Duke, 25 Jan. 2005. Web. 17 Dec. 2015. <https://labs.cellbio.duke.edu/kinesin/KinesinStructure.html>.</li>
<li id="cite_note-5">[[#cite_ref-5|↑]] Rayment I, Rypniewski WR, Schmidt-Base K, Smith R, Tomchick DR, Benning MM, Winkelmann DA, Wesenberg G, Holden HM. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993 Jul 2;261(5117):50-8. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/8316857 8316857]</li>
<li id="cite_note-6">[[#cite_ref-6|↑]] Krukau A, Knecht V, Lipowsky R. Allosteric control of kinesin's motor domain by tubulin: a molecular dynamics study. Phys Chem Chem Phys. 2014 Apr 7;16(13):6189-98. doi: 10.1039/c3cp53367k. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/24561904 24561904] doi:[http://dx.doi.org/10.1039/c3cp53367k http://dx.doi.org/10.1039/c3cp53367k]</li>