This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Cyclin
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
== Structural highlights == | == Structural highlights == | ||
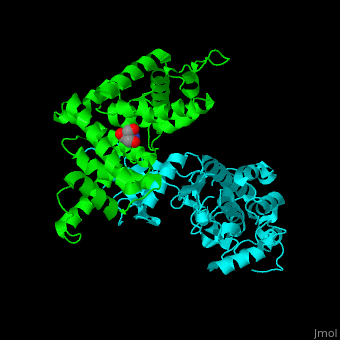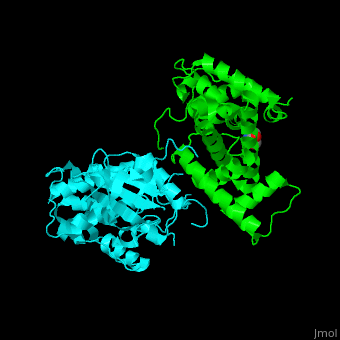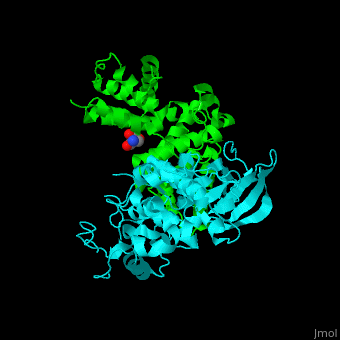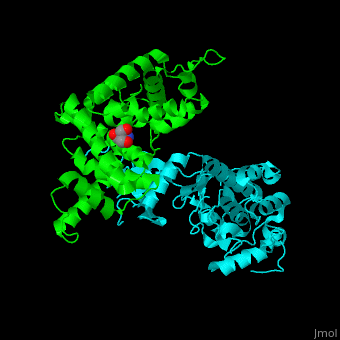
| - | All cyclins have an all-α helix fold and share an identical ca. 100 residue domain called 'cyclin box' which binds CDK. Two 5 α-helix cyclin boxes are shown.<ref>PMID:09433129</ref> | + | All cyclins have an all-α helix fold and share an identical ca. 100 residue domain called 'cyclin box' which binds CDK. <scene name='44/442748/Cv/3'>Two 5 α-helix cyclin boxes are shown</scene>.<ref>PMID:09433129</ref> |
</StructureSection> | </StructureSection> | ||
Revision as of 13:04, 21 December 2015
| |||||||||||
3D Structures of Cyclin
Updated on 21-December-2015
References
- ↑ Galderisi U, Jori FP, Giordano A. Cell cycle regulation and neural differentiation. Oncogene. 2003 Aug 11;22(33):5208-19. PMID:12910258 doi:http://dx.doi.org/10.1038/sj.onc.1206558
- ↑ Noble ME, Endicott JA, Brown NR, Johnson LN. The cyclin box fold: protein recognition in cell-cycle and transcription control. Trends Biochem Sci. 1997 Dec;22(12):482-7. PMID:9433129