We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Cytochrome P450
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
== Structural highlights == | == Structural highlights == | ||
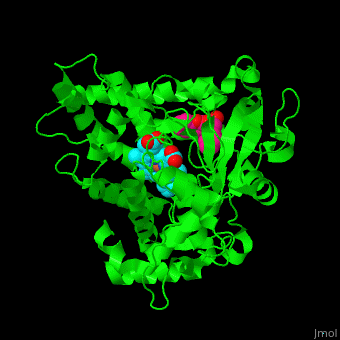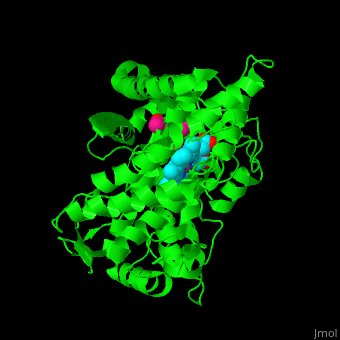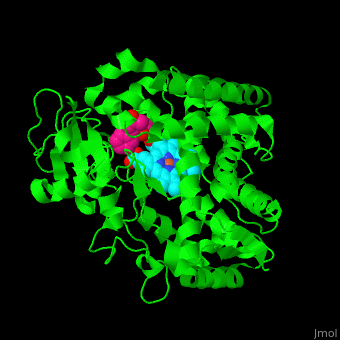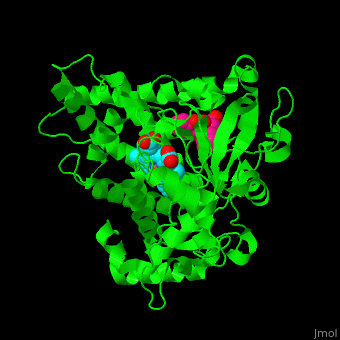
| - | The heme moiety is stabilized by several side chains. The heme iron is pentacoordinated with Cys as one ligand. | + | The <scene name='43/430105/Cv/4'>heme moiety is stabilized by several side chains</scene>. The heme iron is pentacoordinated with Cys as one ligand. |
</StructureSection> | </StructureSection> | ||
== 3D Structures of Cytochrome P450 == | == 3D Structures of Cytochrome P450 == | ||
Revision as of 14:40, 23 December 2015
| |||||||||||
3D Structures of Cytochrome P450
Updated on 23-December-2015
References
- ↑ Danielson PB. The cytochrome P450 superfamily: biochemistry, evolution and drug metabolism in humans. Curr Drug Metab. 2002 Dec;3(6):561-97. PMID:12369887