Vitis vinifera Flavonoid 3-O-Glucosyltransferase (Vv3GT)
From Proteopedia
(Difference between revisions)
| Line 42: | Line 42: | ||
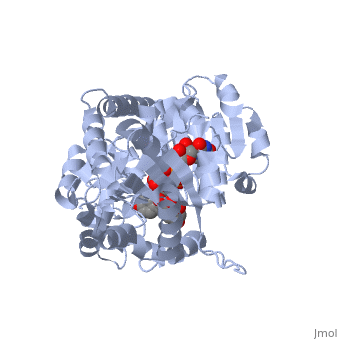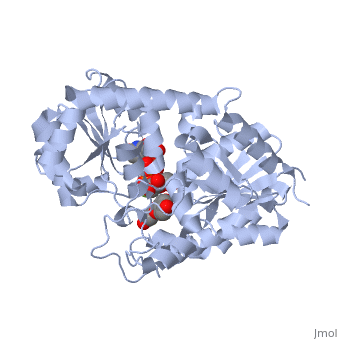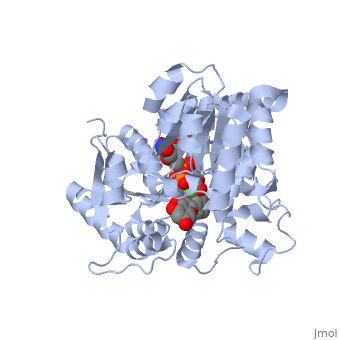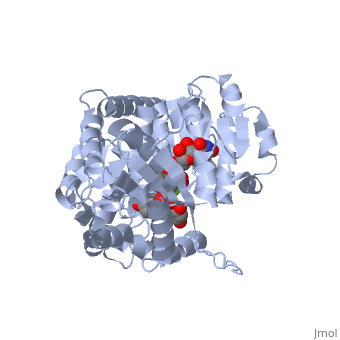
Three conserved motifs involved in sugar binding are present in Vv3GT. The first, a <scene name='60/607848/2c1z_loop_n5_label/1'>loopN5</scene> motif (Thr 141; Ala 142) involved in sugar binding. The second, a <scene name='69/692252/2c1z_wns_label/2'>WNS</scene> (Trp 353; Asn 354; Ser 355) motif residues are involved in binding UDP phosphates. The third, <scene name='69/692252/2c1z_d_eq_label/1'>D/EQ</scene> motif residues also involved in sugar binding (Asp 374; Gln 375). The WNS and D/EQ motifs are part of the highly conserved PSPG region. | Three conserved motifs involved in sugar binding are present in Vv3GT. The first, a <scene name='60/607848/2c1z_loop_n5_label/1'>loopN5</scene> motif (Thr 141; Ala 142) involved in sugar binding. The second, a <scene name='69/692252/2c1z_wns_label/2'>WNS</scene> (Trp 353; Asn 354; Ser 355) motif residues are involved in binding UDP phosphates. The third, <scene name='69/692252/2c1z_d_eq_label/1'>D/EQ</scene> motif residues also involved in sugar binding (Asp 374; Gln 375). The WNS and D/EQ motifs are part of the highly conserved PSPG region. | ||
| + | |||
| + | == 3D structure of glucosyltransferase == | ||
| + | |||
| + | [[Glycosyltransferase]] | ||
== Quiz == | == Quiz == | ||
Revision as of 08:59, 11 January 2016
| |||||||||||
References
- ↑ Yonekura-Sakakibara K, Hanada K. An evolutionary view of functional diversity in family 1 glycosyltransferases. Plant J. 2011 Apr;66(1):182-93. doi: 10.1111/j.1365-313X.2011.04493.x. PMID:21443631 doi:http://dx.doi.org/10.1111/j.1365-313X.2011.04493.x
- ↑ Frydman A, Weisshaus O, Bar-Peled M, Huhman DV, Sumner LW, Marin FR, Lewinsohn E, Fluhr R, Gressel J, Eyal Y. Citrus fruit bitter flavors: isolation and functional characterization of the gene Cm1,2RhaT encoding a 1,2 rhamnosyltransferase, a key enzyme in the biosynthesis of the bitter flavonoids of citrus. Plant J. 2004 Oct;40(1):88-100. PMID:15361143 doi:http://dx.doi.org/10.1111/j.1365-313X.2004.02193.x
- ↑ Osmani SA, Bak S, Moller BL. Substrate specificity of plant UDP-dependent glycosyltransferases predicted from crystal structures and homology modeling. Phytochemistry. 2009 Feb;70(3):325-47. doi: 10.1016/j.phytochem.2008.12.009. Epub, 2009 Feb 13. PMID:19217634 doi:http://dx.doi.org/10.1016/j.phytochem.2008.12.009
- ↑ Osmani SA, Bak S, Moller BL. Substrate specificity of plant UDP-dependent glycosyltransferases predicted from crystal structures and homology modeling. Phytochemistry. 2009 Feb;70(3):325-47. doi: 10.1016/j.phytochem.2008.12.009. Epub, 2009 Feb 13. PMID:19217634 doi:http://dx.doi.org/10.1016/j.phytochem.2008.12.009
- ↑ Offen W, Martinez-Fleites C, Yang M, Kiat-Lim E, Davis BG, Tarling CA, Ford CM, Bowles DJ, Davies GJ. Structure of a flavonoid glucosyltransferase reveals the basis for plant natural product modification. EMBO J. 2006 Mar 22;25(6):1396-405. Epub 2006 Feb 16. PMID:16482224