This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
1bm0
From Proteopedia
| Line 7: | Line 7: | ||
|ACTIVITY= | |ACTIVITY= | ||
|GENE= | |GENE= | ||
| + | |DOMAIN= | ||
| + | |RELATEDENTRY= | ||
| + | |RESOURCES=<span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1bm0 FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1bm0 OCA], [http://www.ebi.ac.uk/pdbsum/1bm0 PDBsum], [http://www.rcsb.org/pdb/explore.do?structureId=1bm0 RCSB]</span> | ||
}} | }} | ||
| Line 14: | Line 17: | ||
==Overview== | ==Overview== | ||
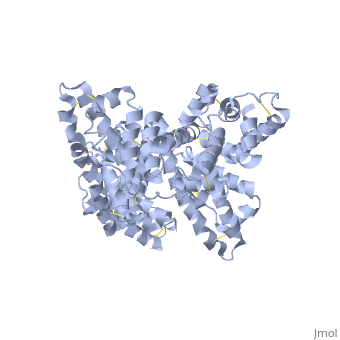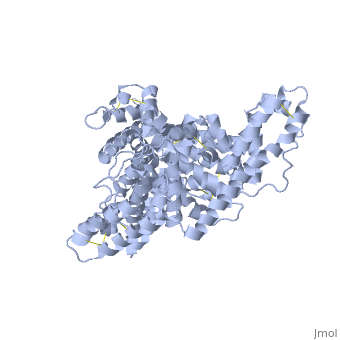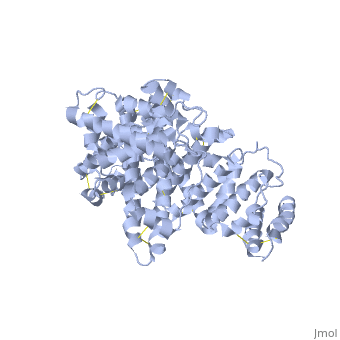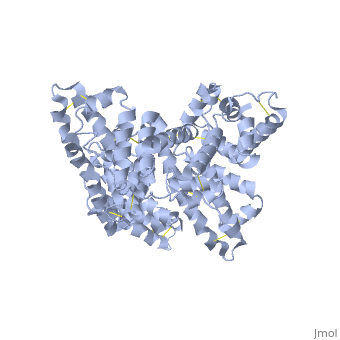
A new triclinic crystal form of human serum albumin (HSA), derived either from pool plasma (pHSA) or from a Pichia pastoris expression system (rHSA), was obtained from polyethylene glycol 4000 solution. Three-dimensional structures of pHSA and rHSA were determined at 2.5 A resolution from the new triclinic crystal form by molecular replacement, using atomic coordinates derived from a multiple isomorphous replacement work with a known tetragonal crystal form. The structures of pHSA and rHSA are virtually identical, with an r.m. s. deviation of 0.24 A for all Calpha atoms. The two HSA molecules involved in the asymmetric unit are related by a strict local twofold symmetry such that the Calpha atoms of the two molecules can be superimposed with an r.m.s. deviation of 0.28 A in pHSA. Cys34 is the only cysteine with a free sulfhydryl group which does not participate in a disulfide linkage with any external ligand. Domains II and III both have a pocket formed mostly of hydrophobic and positively charged residues and in which a very wide range of compounds may be accommodated. Three tentative binding sites for long-chain fatty acids, each with different surroundings, are located at the surface of each domain. | A new triclinic crystal form of human serum albumin (HSA), derived either from pool plasma (pHSA) or from a Pichia pastoris expression system (rHSA), was obtained from polyethylene glycol 4000 solution. Three-dimensional structures of pHSA and rHSA were determined at 2.5 A resolution from the new triclinic crystal form by molecular replacement, using atomic coordinates derived from a multiple isomorphous replacement work with a known tetragonal crystal form. The structures of pHSA and rHSA are virtually identical, with an r.m. s. deviation of 0.24 A for all Calpha atoms. The two HSA molecules involved in the asymmetric unit are related by a strict local twofold symmetry such that the Calpha atoms of the two molecules can be superimposed with an r.m.s. deviation of 0.28 A in pHSA. Cys34 is the only cysteine with a free sulfhydryl group which does not participate in a disulfide linkage with any external ligand. Domains II and III both have a pocket formed mostly of hydrophobic and positively charged residues and in which a very wide range of compounds may be accommodated. Three tentative binding sites for long-chain fatty acids, each with different surroundings, are located at the surface of each domain. | ||
| - | |||
| - | ==Disease== | ||
| - | Known diseases associated with this structure: Analbuminemia OMIM:[[http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=103600 103600]], Dysalbuminemic hyperthyroxinemia OMIM:[[http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=103600 103600]], Dysalbuminemic hyperzincemia OMIM:[[http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=103600 103600]] | ||
==About this Structure== | ==About this Structure== | ||
| Line 33: | Line 33: | ||
[[Category: carrier protein]] | [[Category: carrier protein]] | ||
| - | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Sun Mar 30 19:03:44 2008'' |
Revision as of 16:03, 30 March 2008
| |||||||
| , resolution 2.50Å | |||||||
|---|---|---|---|---|---|---|---|
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||
CRYSTAL STRUCTURE OF HUMAN SERUM ALBUMIN
Overview
A new triclinic crystal form of human serum albumin (HSA), derived either from pool plasma (pHSA) or from a Pichia pastoris expression system (rHSA), was obtained from polyethylene glycol 4000 solution. Three-dimensional structures of pHSA and rHSA were determined at 2.5 A resolution from the new triclinic crystal form by molecular replacement, using atomic coordinates derived from a multiple isomorphous replacement work with a known tetragonal crystal form. The structures of pHSA and rHSA are virtually identical, with an r.m. s. deviation of 0.24 A for all Calpha atoms. The two HSA molecules involved in the asymmetric unit are related by a strict local twofold symmetry such that the Calpha atoms of the two molecules can be superimposed with an r.m.s. deviation of 0.28 A in pHSA. Cys34 is the only cysteine with a free sulfhydryl group which does not participate in a disulfide linkage with any external ligand. Domains II and III both have a pocket formed mostly of hydrophobic and positively charged residues and in which a very wide range of compounds may be accommodated. Three tentative binding sites for long-chain fatty acids, each with different surroundings, are located at the surface of each domain.
About this Structure
1BM0 is a Single protein structure of sequence from Homo sapiens. Full crystallographic information is available from OCA.
Reference
Crystal structure of human serum albumin at 2.5 A resolution., Sugio S, Kashima A, Mochizuki S, Noda M, Kobayashi K, Protein Eng. 1999 Jun;12(6):439-46. PMID:10388840
Page seeded by OCA on Sun Mar 30 19:03:44 2008