Cholera toxin
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
== Structure == | == Structure == | ||
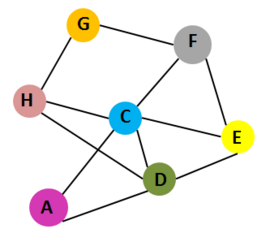
| - | [[Image:CTX interaction.PNG|left|270px|thumb| Interaction of 7 chains of Cholera toxin [[1xtc]]. Cholera toxin contains 7 chains: A,C,D,E,F,G and H.Chains A and C belong to subunit A. Chains | + | [[Image:CTX interaction.PNG|left|270px|thumb| Interaction of 7 chains of Cholera toxin [[1xtc]]. Cholera toxin contains 7 chains: A,C,D,E,F,G and H.Chains A and C belong to subunit A. Chains D,E,F,G and H belong to subunit B]] |
{{Clear}} | {{Clear}} | ||
| - | Cholera toxin(CTX) has two types of subunits: subunit A and subunit B. A subunit contains A1 domain, which includes the enzymatic active site, and A2 domain, which has a α–helix tail. The B subunit contains five chains that form a pentameric ring around the central pore in structure; Subunit A and subunit B are assembled by the α–helix tail of A2 domain, which inserts into the central pore. CTX is the main virulence factor of the pathogen ''Vibrio cholerae'' and cause the major symptom of infection: extreme diarrhea, vomiting, cramps and even death <ref>Ryan KJ; Ray CG (editors) (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. p. 375. ISBN 0838585299.</ref>,<ref>Faruque SM; Nair GB (editors). (2008). Vibrio cholerae: Genomics and Molecular Biology. Caister Academic Press. ISBN 978-1-904455-33-2.</ref>,<ref>Jennifer McDowall, Cholera Toxin, EMBL-EMI, Interpro</ref>. | + | Cholera toxin (CTX) has two types of subunits: subunit A and subunit B. <scene name='43/430104/Cv/3'>A subunit</scene> contains A1 domain, which includes the enzymatic active site, and A2 domain, which has a α–helix tail. The <scene name='43/430104/Cv/4'>B subunit</scene> contains <scene name='43/430104/Cv/5'>five chains that form a pentameric ring around the central pore</scene> in structure; Subunit A and subunit B are assembled by the α–helix tail of A2 domain, which inserts into the central pore. CTX is the main virulence factor of the pathogen ''Vibrio cholerae'' and cause the major symptom of infection: extreme diarrhea, vomiting, cramps and even death <ref>Ryan KJ; Ray CG (editors) (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. p. 375. ISBN 0838585299.</ref>,<ref>Faruque SM; Nair GB (editors). (2008). Vibrio cholerae: Genomics and Molecular Biology. Caister Academic Press. ISBN 978-1-904455-33-2.</ref>,<ref>Jennifer McDowall, Cholera Toxin, EMBL-EMI, Interpro</ref>. |
The enzymatic subunit has a globular domain (CTA1) and a long helical domain (CTA2). Once the CTX binds to the cell surface, it is internalized, and its CTA1 domain binds to ADP-ribosylation factor 6(Arf6)[http://en.wikipedia.org/wiki/ADP_ribosylation_factor], enabling its catalytic activity. | The enzymatic subunit has a globular domain (CTA1) and a long helical domain (CTA2). Once the CTX binds to the cell surface, it is internalized, and its CTA1 domain binds to ADP-ribosylation factor 6(Arf6)[http://en.wikipedia.org/wiki/ADP_ribosylation_factor], enabling its catalytic activity. | ||
Revision as of 13:18, 18 January 2016
| |||||||||||
Contents |
External Links
PDB ID: 1XTC [1]
MMDB ID: 52036 [2]
PubMed[3]
3D Structures of Cholera toxin
Updated on 18-January-2016
See also Toxins
Reference
- ↑ Ryan KJ; Ray CG (editors) (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. p. 375. ISBN 0838585299.
- ↑ Faruque SM; Nair GB (editors). (2008). Vibrio cholerae: Genomics and Molecular Biology. Caister Academic Press. ISBN 978-1-904455-33-2.
- ↑ Jennifer McDowall, Cholera Toxin, EMBL-EMI, Interpro
- ↑ Jennifer McDowall, Cholera Toxin, EMBL-EMI, Interpro
- ↑ Davis B, Waldor M (2003). "Filamentous phages linked to virulence of Vibrio cholerae". Curr Opin Microbiol 6 (1): 35–42. doi:10.1016/S1369-5274(02)00005-X. PMID 12615217.
- ↑ Luppi P.H.. "The Discovery of Cholera-Toxin as a Powerful Neuroanatomical Tool". Retrieved 2011-03-23.
- ↑ Luppi P.H., Fort P., Jouvet M. Iontophoretic application of unconjugated cholera toxin B subunit (CTb) combined with immunohistochemistry of neurochemical substances: a method for transmitter identification of retrogradely labeled neurons. Brain Res. 534 (1-2) pages : 209-224 (1990)