We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Ferritin
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
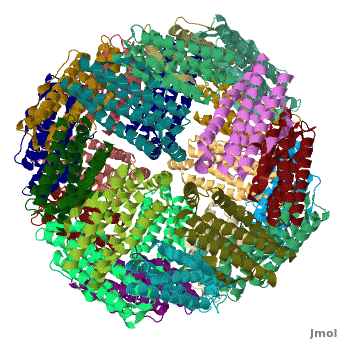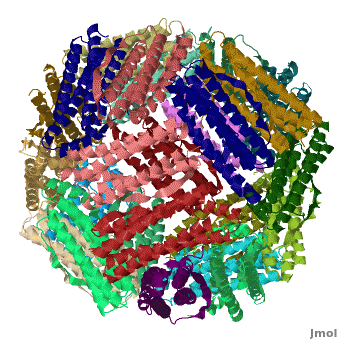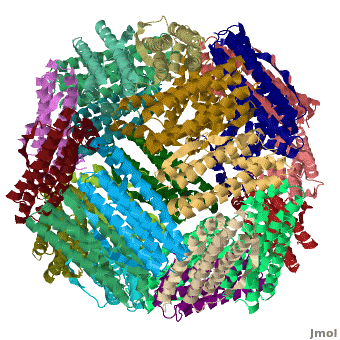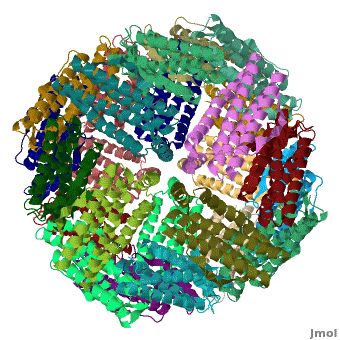
<applet load='Pdb2jd7.pdb' size='400' frame='true' align='right' scene="Ferritin/Starting_ferritin_scene/1" caption="Crystal structure of Fe-soaked ferritin from the hyperthermophilic archael anaerobe ''Pyrococcus furiousus'', [[2jd7]]" /> | <applet load='Pdb2jd7.pdb' size='400' frame='true' align='right' scene="Ferritin/Starting_ferritin_scene/1" caption="Crystal structure of Fe-soaked ferritin from the hyperthermophilic archael anaerobe ''Pyrococcus furiousus'', [[2jd7]]" /> | ||
| + | == Function == | ||
| - | [[Ferritin]] (FR) is an iron storage and release protein. It stores iron as microcrystals with phosphate and hydroxide ions. FR is composed of 24 subunits of heavy chain (FTH) | + | [[Ferritin]] (FR) is an iron storage and release protein. It stores iron as microcrystals with phosphate and hydroxide ions. FR is composed of 24 subunits of heavy chain (FTH) a<ref>PMID:20304033</ref> |
| + | |||
| + | nd light chain (FTL). Amphibians have an additional middle subunit FR (FTM).<br /> | ||
* '''Bacterioferritin''' (BFR) structure is very similar to FR. It contains a binuclear iron center and haem. It stores iron as ferric oxide mineral in its hollow central cavity.<br /> | * '''Bacterioferritin''' (BFR) structure is very similar to FR. It contains a binuclear iron center and haem. It stores iron as ferric oxide mineral in its hollow central cavity.<br /> | ||
* '''Thioferritin''' (TFR) is a ferritin-related protein with 2 cysteine residues adjacent to the dimetal binding site.<br /> | * '''Thioferritin''' (TFR) is a ferritin-related protein with 2 cysteine residues adjacent to the dimetal binding site.<br /> | ||
* Another iron storing protein is the '''DNA-binding Protein of Starved cells''' (Dps) - a ferritin-like diiron carboxylate.<br /> | * Another iron storing protein is the '''DNA-binding Protein of Starved cells''' (Dps) - a ferritin-like diiron carboxylate.<br /> | ||
* '''MrgA''' – another iron storage protein - belongs to the Dps family. | * '''MrgA''' – another iron storage protein - belongs to the Dps family. | ||
| + | |||
| + | == Relevance == | ||
| + | |||
| + | Cavities formed by FR are used for the manufacture of nanoparticles. FR is used as a marker for iron overload disorder. | ||
| + | |||
| + | == Disease == | ||
| + | |||
| + | FR deficiency can lead to anemia. | ||
==3D Structures of Ferritin== | ==3D Structures of Ferritin== | ||
| Line 144: | Line 155: | ||
**[[2chp]] – MrgA – ''Bacillus subtilis''<br /> | **[[2chp]] – MrgA – ''Bacillus subtilis''<br /> | ||
}} | }} | ||
| + | == References == | ||
| + | <references/> | ||
| + | |||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Revision as of 20:21, 21 January 2016
|
Contents |
Function
Ferritin (FR) is an iron storage and release protein. It stores iron as microcrystals with phosphate and hydroxide ions. FR is composed of 24 subunits of heavy chain (FTH) a[1]
nd light chain (FTL). Amphibians have an additional middle subunit FR (FTM).
- Bacterioferritin (BFR) structure is very similar to FR. It contains a binuclear iron center and haem. It stores iron as ferric oxide mineral in its hollow central cavity.
- Thioferritin (TFR) is a ferritin-related protein with 2 cysteine residues adjacent to the dimetal binding site.
- Another iron storing protein is the DNA-binding Protein of Starved cells (Dps) - a ferritin-like diiron carboxylate.
- MrgA – another iron storage protein - belongs to the Dps family.
Relevance
Cavities formed by FR are used for the manufacture of nanoparticles. FR is used as a marker for iron overload disorder.
Disease
FR deficiency can lead to anemia.
3D Structures of Ferritin
Updated on 21-January-2016
References
- ↑ Wang W, Knovich MA, Coffman LG, Torti FM, Torti SV. Serum ferritin: Past, present and future. Biochim Biophys Acta. 2010 Aug;1800(8):760-9. doi: 10.1016/j.bbagen.2010.03.011. , Epub 2010 Mar 19. PMID:20304033 doi:http://dx.doi.org/10.1016/j.bbagen.2010.03.011
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Joel L. Sussman, David Canner, Eran Hodis, Alexander Berchansky