We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1122
From Proteopedia
(Difference between revisions)
| Line 14: | Line 14: | ||
=== IP3R inhibition === | === IP3R inhibition === | ||
| - | Bcl-2 localized at the endoplasmic reticulum (ER) membranes participates in the control of Ca2+ content and release. The inositol 1,4,5-trisphosphate receptor (IP3R) | + | Bcl-2 localized at the endoplasmic reticulum (ER) membranes participates in the control of Ca2+ content and release. The inositol 1,4,5-trisphosphate receptor (IP3R) is the primary Ca2+ release channel localized in the ER. Its pro-apoptotic activity can be directly inhibited by the Bcl-2, homology domain 4 (BH4) being essential and sufficient for this effect. BH4 comprises 20 amino acids (10-30) organized in alpha-helical structure which is required to inhibit IP3R. Residues K17, H20, Y21 and R26 participate in the inhibition of IP3R because they are very accessible and proximal in the secondary structure. <ref> [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3795776/ Alpha-Helical Destabilization of the Bcl-2-BH4-Domain Peptide Abolishes Its Ability to Inhibit the IP3 Receptor]</ref> |
=== Regulation of the mitochondrial pathway of apoptosis === | === Regulation of the mitochondrial pathway of apoptosis === | ||
| Line 26: | Line 26: | ||
Therefore, the neutralization of Bcl-2 is required for eficient cell-death. BH3 proteins Bad, Bim and Puma bind Bcl-2 and disable its anti-apoptotic activity. BH3 peptides occupy the hydrophobic pocket of Bcl-2 and the sequestered pro-apoptotic proteins are released. <ref>[http://www.cell.com/molecular-cell/fulltext/S1097-2765(05)01040-3 Differential Targeting of Prosurvival Bcl-2 Proteins by Their BH3-Only Ligands Allows Complementary Apoptotic Function]</ref> <ref> [http://www.cell.com/cancer-cell/fulltext/S1535-6108(02)00127-7 Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics] </ref> | Therefore, the neutralization of Bcl-2 is required for eficient cell-death. BH3 proteins Bad, Bim and Puma bind Bcl-2 and disable its anti-apoptotic activity. BH3 peptides occupy the hydrophobic pocket of Bcl-2 and the sequestered pro-apoptotic proteins are released. <ref>[http://www.cell.com/molecular-cell/fulltext/S1097-2765(05)01040-3 Differential Targeting of Prosurvival Bcl-2 Proteins by Their BH3-Only Ligands Allows Complementary Apoptotic Function]</ref> <ref> [http://www.cell.com/cancer-cell/fulltext/S1535-6108(02)00127-7 Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics] </ref> | ||
| - | Bcl-2 can also inhibit the release of cytochrome c from mitochondria | + | Bcl-2 localized on external mitochondrial membrane can also inhibit the release of cytochrome c from mitochondria. <ref>[http://science.sciencemag.org/content/275/5303/1132.full The Release of Cytochrome c from Mitochondria: A Primary Site for Bcl-2 Regulation of Apoptosis] </ref> <ref>[http://science.sciencemag.org/content/275/5303/1129.full Prevention of Apoptosis by Bcl-2: Release of Cytochrome c from Mitochondria Blocked] </ref> |
== Disease == | == Disease == | ||
Revision as of 14:53, 28 January 2016
| This Sandbox is Reserved from 15/12/2015, through 15/06/2016 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1120 through Sandbox Reserved 1159. |
To get started:
More help: Help:Editing |
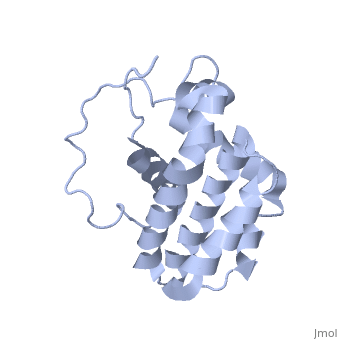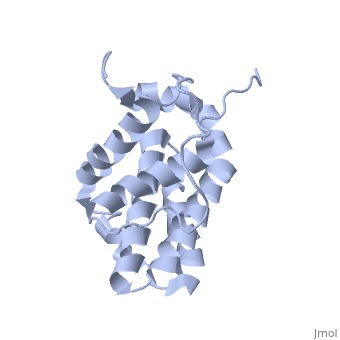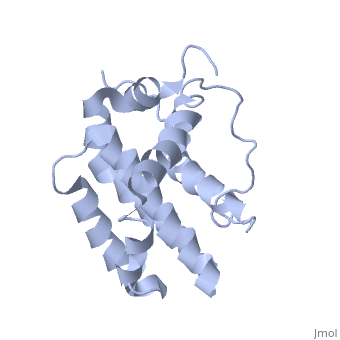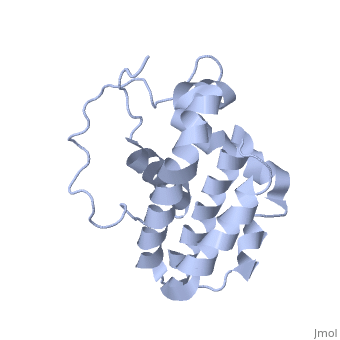
HUMAN BCL-2, ISOFORM1
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ Alpha-Helical Destabilization of the Bcl-2-BH4-Domain Peptide Abolishes Its Ability to Inhibit the IP3 Receptor
- ↑ Control of mitochondrial apoptosis by the Bcl-2 family
- ↑ Differential Targeting of Prosurvival Bcl-2 Proteins by Their BH3-Only Ligands Allows Complementary Apoptotic Function
- ↑ Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics
- ↑ The Release of Cytochrome c from Mitochondria: A Primary Site for Bcl-2 Regulation of Apoptosis
- ↑ Prevention of Apoptosis by Bcl-2: Release of Cytochrome c from Mitochondria Blocked