We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1122
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
{{Sandbox_Reserved_ESBS_2015}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | {{Sandbox_Reserved_ESBS_2015}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
==HUMAN BCL-2, ISOFORM1== | ==HUMAN BCL-2, ISOFORM1== | ||
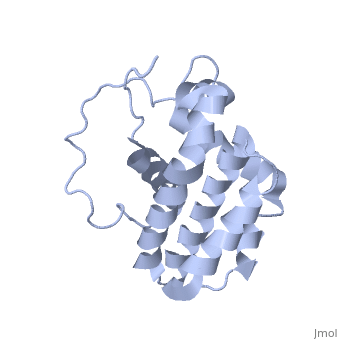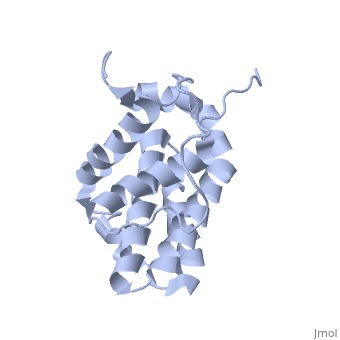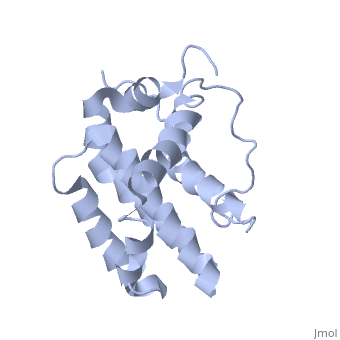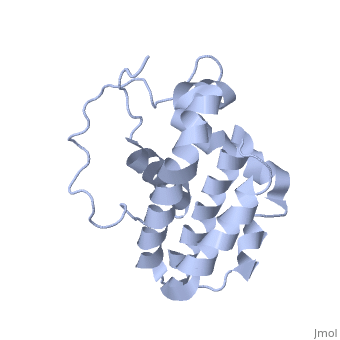
| - | <StructureSection load='1g5m' size='400' side='right' caption='HUMAN BCL-2, ISOFORM1' scene=''> | + | <StructureSection load='1g5m' size='400' side='right' caption='3D STRUCTURE OF HUMAN BCL-2, ISOFORM1 (from residue 3 to 207) BASED ON NMR SPECTROSCOPY' scene=''> |
Human BCL-2, isoform 1 is an oncoprotein of 239 residues regulating cell death (apoptosis), notably acting as an anti-apoptotic. It is encoded by the BCL2 gene located on the 18th chromosome (63.12-63.32 Mb). There are 2 isoforms of this protein (𝛼 and 𝛽), produced by alternative splicing, and which differ by 2 aminoacids (residues 96 (A→T) and 110 (R→G))(ref). Alteration of this protein is a caused of many cancers, and is also likely to be involved in schyzophrenia and autoimmunity. | Human BCL-2, isoform 1 is an oncoprotein of 239 residues regulating cell death (apoptosis), notably acting as an anti-apoptotic. It is encoded by the BCL2 gene located on the 18th chromosome (63.12-63.32 Mb). There are 2 isoforms of this protein (𝛼 and 𝛽), produced by alternative splicing, and which differ by 2 aminoacids (residues 96 (A→T) and 110 (R→G))(ref). Alteration of this protein is a caused of many cancers, and is also likely to be involved in schyzophrenia and autoimmunity. | ||
| Line 10: | Line 10: | ||
Human BCL-2, isoform 1 is a 26kDa protein of 239 residues. The linear structure highlights 5 domains: <scene name='71/719863/Scenelucas/1'>BH4</scene> (10-30), | Human BCL-2, isoform 1 is a 26kDa protein of 239 residues. The linear structure highlights 5 domains: <scene name='71/719863/Scenelucas/1'>BH4</scene> (10-30), | ||
<scene name='71/719863/Scenebh3/2'>BH3</scene> (93-107), <scene name='71/719863/Scenebh1/1'>BH1</scene> (136-155), <scene name='71/719863/Scenebh2/1'>BH2</scene> (187-202) and a | <scene name='71/719863/Scenebh3/2'>BH3</scene> (93-107), <scene name='71/719863/Scenebh1/1'>BH1</scene> (136-155), <scene name='71/719863/Scenebh2/1'>BH2</scene> (187-202) and a | ||
| - | + | transmembrane domain (218-239). It organizes as eight alpha-helices: from 11 to 25 (1) , from 93 to 107 (2), from 109 to 118 (3), from 126 to 137 (4), from 144-163 (5), from 169 to 184 (6), from 186 to 191 (7) and from 194 to 202(8). There are also 3 turns (32-34, 123-125, 138-140). The 3rd alpha-helix is a 3(10) helix, whereas BCL-XL 3rd helix is a normal alpha-helix. | |
== Function == | == Function == | ||
Revision as of 15:26, 28 January 2016
| This Sandbox is Reserved from 15/12/2015, through 15/06/2016 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1120 through Sandbox Reserved 1159. |
To get started:
More help: Help:Editing |
HUMAN BCL-2, ISOFORM1
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ Alpha-Helical Destabilization of the Bcl-2-BH4-Domain Peptide Abolishes Its Ability to Inhibit the IP3 Receptor
- ↑ Control of mitochondrial apoptosis by the Bcl-2 family
- ↑ Differential Targeting of Prosurvival Bcl-2 Proteins by Their BH3-Only Ligands Allows Complementary Apoptotic Function
- ↑ Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics
- ↑ The Release of Cytochrome c from Mitochondria: A Primary Site for Bcl-2 Regulation of Apoptosis
- ↑ Prevention of Apoptosis by Bcl-2: Release of Cytochrome c from Mitochondria Blocked