Sandbox Reserved 1121
From Proteopedia
| Line 20: | Line 20: | ||
=== Ca<sup>2+</sup>-binding site === | === Ca<sup>2+</sup>-binding site === | ||
| - | [[Image:Avec ca2+.PNG | thumb | Loop involved in Ca<sup>2+</sup>-binding site <ref name="PyMol">The PyMOL Molecular Graphics System, Version 1.8 Schrödinger, LLC.</ref>][Image:Sans Ca2+.PNG | thumb | | + | [[Image:Avec ca2+.PNG | thumb | Loop involved in Ca<sup>2+</sup>-binding site <ref name="PyMol">The PyMOL Molecular Graphics System, Version 1.8 Schrödinger, LLC.</ref>]][[Image:Sans Ca2+.PNG | thumb | Ca<sup>2+</sup>-depleted structure <ref name="PyMol"/>]] |
CRP is a calcium dependent structure. The face B binds two Ca<sup>2+</sup> ions per subunit. The ligands are bound asymmetrically in both Ca<sup>2+</sup>-binding sites. The residues of the site 1 include Asp60, Asn61, Glu138, Asp140, the carbonyl oxygen of the residue 139 and one oxygen of Asp60. The site 1 provides a total of five ligands for the Ca<sup>2+</sup> ion. The site 2 residues include Gln138, Asp140, Gln150 and non-coordinating Glu147. The site 2 provides four ligands for the Ca<sup>2+</sup> ion. The two Ca<sup>2+</sup>-binding sites are of equal affinity for Ca<sup>2+</sup> and are only differentiated by the carbonyl oxygen ligand. In presence of Ca<sup>2+</sup>, the two Ca<sup>2+</sup>-binding sites are overlapping in a loop. When the Ca<sup>2+</sup> sites are free, the residues 140 to 150 form a loop away from the molecule<ref name="Thompson"/>. This releases the proteolysis site <ref name="agrawal"/>, which induces the cleavage of CRP between Asn145 and Phe146 by nagarase protease and between Phe146 and Glu147 by pronase <ref name="ramadan">PMID: 12037301</ref>. Therefore Ca<sup>2+</sup> protects CRP form proteolytic cleavage and from degradation <ref name="agrawal"/>. | CRP is a calcium dependent structure. The face B binds two Ca<sup>2+</sup> ions per subunit. The ligands are bound asymmetrically in both Ca<sup>2+</sup>-binding sites. The residues of the site 1 include Asp60, Asn61, Glu138, Asp140, the carbonyl oxygen of the residue 139 and one oxygen of Asp60. The site 1 provides a total of five ligands for the Ca<sup>2+</sup> ion. The site 2 residues include Gln138, Asp140, Gln150 and non-coordinating Glu147. The site 2 provides four ligands for the Ca<sup>2+</sup> ion. The two Ca<sup>2+</sup>-binding sites are of equal affinity for Ca<sup>2+</sup> and are only differentiated by the carbonyl oxygen ligand. In presence of Ca<sup>2+</sup>, the two Ca<sup>2+</sup>-binding sites are overlapping in a loop. When the Ca<sup>2+</sup> sites are free, the residues 140 to 150 form a loop away from the molecule<ref name="Thompson"/>. This releases the proteolysis site <ref name="agrawal"/>, which induces the cleavage of CRP between Asn145 and Phe146 by nagarase protease and between Phe146 and Glu147 by pronase <ref name="ramadan">PMID: 12037301</ref>. Therefore Ca<sup>2+</sup> protects CRP form proteolytic cleavage and from degradation <ref name="agrawal"/>. | ||
Revision as of 15:37, 28 January 2016
|
| This Sandbox is Reserved from 15/12/2015, through 15/06/2016 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1120 through Sandbox Reserved 1159. |
To get started:
More help: Help:Editing |
Contents |
Human C-reactive protein complexed with phosphocholine
Human C-Reactive Protein (CRP) is an acute phase protein belonging to the highly conserved pentraxin protein family, like as its homologue, the Serum Amyloid P component (SAP) [1]. Although it is a normal serum protein, its circulating concentration raises rapidly and extensively in a cytokine-mediated in response to an infection, an inflammation or a tissue injury. In this way, serum CRP rate is empirically dose to detect many human disease [1]. CRP was named this way because it was found that the protein could precipitate the "C" polysaccharide derived from Streptococcus pneumoniae cell wall [2][3].
Structure
CRP structure
The crystal structure of CRP was determined by using SAP as the search model [1]. The structure of CRP has been obtained by X-ray crystallography at 3 Å resolution. Like SAP, the protein consists of five protomers, uncovalently bonded and nonglycosylated that are arranged symmetrically around a central pore [4]. Each subunit is a 25 kDa protein consisting of 224 residues [5]. The diameter of the CRP pentamer is 102 Å, the inner pore diameter is 30 Å and the diameter of a subunit is 36 Å [6][4]. Ser53, His95, Cys97, Asp112, Gly113, Gly136, Gly154, Val165, Leu166, Ile171, and Gly196 are the highliest conserved residues in the primary sequence of CRP [3].
Each subunit consist of two antiparallel [5] with a flattened jellyroll topology [4] and a long (residues 168-176) lies folded against the β-sheets. The predominant structure is β-sheet [7] but short helical regions can be noticed for the residues 43 and 185 [3]. The carboxyl terminal end of the helix along with loop 177-182 form one of the two sides of a cleft that extends from the centre of the promoter to its edge at the central pore of the pentamer [4]. The other side of the cleft is formed by parts of the amino and carboxyl termini of the promoter [4]. This furrow is 24 Å long, 7.5 Å deep and 12.4 Å wide. The side walls are constructed from Ser5, Arg6, Gln203, Pro206, Trp187, Arg188, Asn160, Gly177, Leu176, Tyr175, His95 and Asp112 [1]. This cleft is involved in C1q binding and perhaps also with the FcR binding [4].[8][4].The outer part of the furrow is positively charged but the inner part terminates halfway through the pentamer pore at residue Asp112, providing a ring of negative charges lining the pore [1]. Asp112 seems to be an important residue for recognition of Cq1 by CRP [1] because it is considered, with Tyr175, to be C1q contact residues [4]. Glu88 seems to influence the conformational change of C1q necessary for complement activation, while Asn158 and His38 may contribute to the correct geometry of the binding site [4].
On the other face of the protomer (face A), two calcium ions are bound 4 Å apart by protein sidechains coming from loops collected at the concave face (face B) and this is the site of ligand binding [1]. Each subunit in CRP is rotated by 22° towards the fivefold axis such that the helices of face A are 5 Å closer to the axis and the calcium sites on face B move away by an equivalent amount [4][1]. Thus, CRP pentamer has two faces: a face that exibit the five phosphocholine binding sites and a face involve in the binding of C1q and possibly Fcr [4].
The five protomers are related one with an other by three salt bridges that mainly involve the 115-123 loop of one protomer and the 40-42 and 197-202 regions of the other promoter [4].
Ca2+-binding site
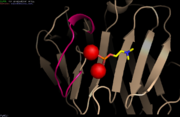

CRP is a calcium dependent structure. The face B binds two Ca2+ ions per subunit. The ligands are bound asymmetrically in both Ca2+-binding sites. The residues of the site 1 include Asp60, Asn61, Glu138, Asp140, the carbonyl oxygen of the residue 139 and one oxygen of Asp60. The site 1 provides a total of five ligands for the Ca2+ ion. The site 2 residues include Gln138, Asp140, Gln150 and non-coordinating Glu147. The site 2 provides four ligands for the Ca2+ ion. The two Ca2+-binding sites are of equal affinity for Ca2+ and are only differentiated by the carbonyl oxygen ligand. In presence of Ca2+, the two Ca2+-binding sites are overlapping in a loop. When the Ca2+ sites are free, the residues 140 to 150 form a loop away from the molecule[1]. This releases the proteolysis site [6], which induces the cleavage of CRP between Asn145 and Phe146 by nagarase protease and between Phe146 and Glu147 by pronase [10]. Therefore Ca2+ protects CRP form proteolytic cleavage and from degradation [6].
Phosphocholine-binding site
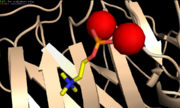
PC stands for phosphocholine. It is a phospholipid in cell membranes and a plasma lipoprotein [1]. The PC-binding site is a hydrophobic pocket constituted by the residues Leu64, Phe66, Thr76 and the two Ca2+ [6]. The choline function of PC interacts with the two key residues Phe66 and Glu81, therefore PC lies inside the PC-binding site [3] [6]. The PC-binding site is next to the Ca2+-binding sites on the same face of the CRP protein. The phosphate groupe of PC interacts by coordination with the two Ca2+ [6]. The affinity of CRP for PC increases with the concentration of PC. A surface containing a high density of PC, such as C-polycaccharide, is therefore propitious to the CRP-binding [8]. CRP can also bind chromatin, histones, small nuclear ribonucleoproteins nuclear envelop proteins and nucleosomes Ca2+-dependently [6].
Function
CRP is involved in the primary and innate host defense. PC is present in teichoic acids, capsular carbohydrates, and lipopolysaccharides of bacteria and other micro-organisms. There are many PC-expressing micro-organisms such as Streptococcus pneumoniae, Haemophilus influenzae, Pseudomonas aeruginosa, Neisseria meningitides and Neisseria gonorrhoeae, Proteus morganii and Aspergillus fumigatus [4]. It binds to PC located on the surface of pathogens bacteria that infected the organism. The resulting immune response is the phagocytosis of PC-expressing bacteria [6]. Moreover, apoptic and necrotic cells which belong to the host organism can be treated by the CRP resulting in a restoration of function in targeted tissues [4]. The CRP is therefore part of the acute phase response which is a rapid concentration variation of plasma proteins [11]. When CRP is bind to a multivalent ligand, C1q is able to recognize it. This mechanism is initiated the C3 convertase assembly, which is an enzyme involved in the innate response. Furthermore, multiple pentamers have to be close to each other in order to realize this assembly [4].
Biomedical interest
Healthy humans have a CRP rate which is generally about 1 μg/mL[3]. CRP is secreted by the liver into the blood circulation[11]. CRP level is 1000 times higher in a cytokine-mediated response due to tissue injury, infection and inflammation. Therefore the CRP rate in serum is common use to detect the activity of a disease[1]. CRP can be defined as a target for the development of cardioprotection and neuroprotection[3].
Structural highlights
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
</StructureSection>
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 Thompson D, Pepys MB, Wood SP. The physiological structure of human C-reactive protein and its complex with phosphocholine. Structure. 1999 Feb 15;7(2):169-77. PMID:10368284
- ↑ Black S, Kushner I, Samols D. C-reactive Protein. J Biol Chem. 2004 Nov 19;279(47):48487-90. Epub 2004 Aug 26. PMID:15337754 doi:http://dx.doi.org/10.1074/jbc.R400025200
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Kumar SV, Ravunny RK, Chakraborty C. Conserved domains, conserved residues, and surface cavities of C-reactive protein (CRP). Appl Biochem Biotechnol. 2011 Sep;165(2):497-505. doi: 10.1007/s12010-011-9270-7., Epub 2011 May 4. PMID:21541851 doi:http://dx.doi.org/10.1007/s12010-011-9270-7
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 4.14 Volanakis JE. Human C-reactive protein: expression, structure, and function. Mol Immunol. 2001 Aug;38(2-3):189-97. PMID:11532280
- ↑ 5.0 5.1 UniProtKB - P02741 (CRP_HUMAN)
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 Agrawal A, Singh PP, Bottazzi B, Garlanda C, Mantovani A. Pattern recognition by pentraxins. Adv Exp Med Biol. 2009;653:98-116. PMID:19799114
- ↑ Dong A, Caughey B, Caughey WS, Bhat KS, Coe JE. Secondary structure of the pentraxin female protein in water determined by infrared spectroscopy: effects of calcium and phosphorylcholine. Biochemistry. 1992 Oct 6;31(39):9364-70. PMID:1382589
- ↑ 8.0 8.1 Du Clos TW, Mold C. C-reactive protein: an activator of innate immunity and a modulator of adaptive immunity. Immunol Res. 2004;30(3):261-77. PMID:15531769 doi:http://dx.doi.org/10.1385/IR:30:3:261
- ↑ 9.0 9.1 9.2 The PyMOL Molecular Graphics System, Version 1.8 Schrödinger, LLC.
- ↑ Ramadan MA, Shrive AK, Holden D, Myles DA, Volanakis JE, DeLucas LJ, Greenhough TJ. The three-dimensional structure of calcium-depleted human C-reactive protein from perfectly twinned crystals. Acta Crystallogr D Biol Crystallogr. 2002 Jun;58(Pt 6 Pt 2):992-1001. Epub, 2002 May 29. PMID:12037301
- ↑ 11.0 11.1 Szalai AJ. The biological functions of C-reactive protein. Vascul Pharmacol. 2002 Aug;39(3):105-7. PMID:12616974
