We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1125
From Proteopedia
(Difference between revisions)
| Line 32: | Line 32: | ||
=== Ca2+ interactions === | === Ca2+ interactions === | ||
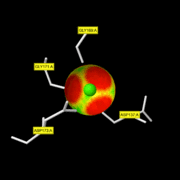
| - | [[Image:CA_pocket_interaction.gif | thumb| | + | [[Image:CA_pocket_interaction.gif | thumb|CA996 pocket interaction]]This enzyme binds 3 Ca ions, 2 of them in the catalytic domain, which are packed against the top of the beta sheet and mostly have a structural function, stabilizing the catalytic domain. |
The residues involved in the Ca996 interactions (coordinate bonds) are <scene name='71/719866/Ca2_interactions/1'>two Gly residues (169 and 171) next to two Asp residues (137 and 173)</scene>. | The residues involved in the Ca996 interactions (coordinate bonds) are <scene name='71/719866/Ca2_interactions/1'>two Gly residues (169 and 171) next to two Asp residues (137 and 173)</scene>. | ||
| Line 39: | Line 39: | ||
==== Zn999 : the catalytic zinc ==== | ==== Zn999 : the catalytic zinc ==== | ||
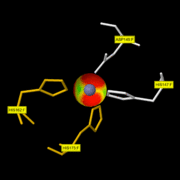
It is involved in the catalytic activity and is situated at the bottom of the active-site. In a publication [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC394940/?page=2] which studies the catalytic domain of MMP8 thanks to the Pro-Leu-Gly-hydroxylamine inhibitor, this ion is penta-coordinated with: His197, His201 and His207 of MMP8 and with the carbonyl and the hydroxyl oxygen of the hydroxamic acid moiety of the inhibitor. On this <scene name='71/719866/Zn999_interactions/2'>link</scene> you can only see the 3 His of MMP8 with the Zn999. The fourth ligand of the catalytic zinc is a water molecule. | It is involved in the catalytic activity and is situated at the bottom of the active-site. In a publication [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC394940/?page=2] which studies the catalytic domain of MMP8 thanks to the Pro-Leu-Gly-hydroxylamine inhibitor, this ion is penta-coordinated with: His197, His201 and His207 of MMP8 and with the carbonyl and the hydroxyl oxygen of the hydroxamic acid moiety of the inhibitor. On this <scene name='71/719866/Zn999_interactions/2'>link</scene> you can only see the 3 His of MMP8 with the Zn999. The fourth ligand of the catalytic zinc is a water molecule. | ||
| - | [[Image:ZN pocket interaction.gif | thumb| | + | [[Image:ZN pocket interaction.gif | thumb|ZN999 pocket interaction]] |
==== Zn998 : the structural zinc ==== | ==== Zn998 : the structural zinc ==== | ||
The residues involved in the Zn998 interactions are <scene name='71/719866/Zn998/1'>an Asp residue (149) next to three His residues (147, 162 and 175)</scene>. The glutamic acid adjacent to the first histidine is essential for catalysis. It's good to know that W Bode and al.[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC394940/?page=2] were unable to exchange or remove this Zinc in their crystals, which is suggesting that there is a tight interaction with MMP-8. | The residues involved in the Zn998 interactions are <scene name='71/719866/Zn998/1'>an Asp residue (149) next to three His residues (147, 162 and 175)</scene>. The glutamic acid adjacent to the first histidine is essential for catalysis. It's good to know that W Bode and al.[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC394940/?page=2] were unable to exchange or remove this Zinc in their crystals, which is suggesting that there is a tight interaction with MMP-8. | ||
Revision as of 18:10, 28 January 2016
MMP8
MMP-8, also called, Neutrophil collagenase or Collagenase 2, is a zinc-dependent and calcium-dependent enzyme. It belongs to the matrix metalloproteinase (MMP) family which is involved in the breakdown of extracellular matrix in embryonic development, reproduction, and tissue remodeling, as well as in disease processes, such as arthritis and metastasis. The gene coding this family is localized on the chromosome 11 of Homo sapiens .[1]
| |||||||||||
References
- ↑ "MMP8 matrix metallopeptidase 8 (neutrophil collagenase)"
- ↑ "Metalloendopeptidase activity"
- ↑ Stams T, Spurlino JC, Smith DL, Wahl RC, Ho TF, Qoronfleh MW, Banks TM, Rubin B. Structure of human neutrophil collagenase reveals large S1' specificity pocket. Nat Struct Biol. 1994 Feb;1(2):119-23. PMID:7656015
- ↑ 4.0 4.1 Substrate specificity of MMPs
- ↑ Hirose T, Patterson C, Pourmotabbed T, Mainardi CL, Hasty KA. Structure-function relationship of human neutrophil collagenase: identification of regions responsible for substrate specificity and general proteinase activity. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2569-73. PMID:8464863
- ↑ Knauper V, Osthues A, DeClerck YA, Langley KE, Blaser J, Tschesche H. Fragmentation of human polymorphonuclear-leucocyte collagenase. Biochem J. 1993 May 1;291 ( Pt 3):847-54. PMID:8489511
- ↑ Welgus HG, Jeffrey JJ, Eisen AZ. Human skin fibroblast collagenase. Assessment of activation energy and deuterium isotope effect with collagenous substrates. J Biol Chem. 1981 Sep 25;256(18):9516-21. PMID:6270090
- ↑ Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003 May 2;92(8):827-39. PMID:12730128 doi:http://dx.doi.org/10.1161/01.RES.0000070112.80711.3D
- ↑ Knauper V, Docherty AJ, Smith B, Tschesche H, Murphy G. Analysis of the contribution of the hinge region of human neutrophil collagenase (HNC, MMP-8) to stability and collagenolytic activity by alanine scanning mutagenesis. FEBS Lett. 1997 Mar 17;405(1):60-4. PMID:9094424
- ↑ "Neutrophil collagenase"
- ↑ "Extra Binding Region Induced by Non-Zinc Chelating Inhibitors into the S1′ Subsite of Matrix Metalloproteinase 8"
- ↑ Balbin M, Fueyo A, Knauper V, Pendas AM, Lopez JM, Jimenez MG, Murphy G, Lopez-Otin C. Collagenase 2 (MMP-8) expression in murine tissue-remodeling processes. Analysis of its potential role in postpartum involution of the uterus. J Biol Chem. 1998 Sep 11;273(37):23959-68. PMID:9727011
RESSOURCE : Image:2oy4 mm1.pdb ( la structure du monomère )