We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1137
From Proteopedia
(Difference between revisions)
| Line 21: | Line 21: | ||
== Structural highlights == | == Structural highlights == | ||
===Atg3 protein=== | ===Atg3 protein=== | ||
| + | ---- | ||
| + | |||
Atg3 is a protein composed of 314 aminoacids with an alpha/beta-fold with a core region topologically similar to E2 enzymes. The core region has two regions: | Atg3 is a protein composed of 314 aminoacids with an alpha/beta-fold with a core region topologically similar to E2 enzymes. The core region has two regions: | ||
→ the first region has 80 residues and has a random coil structure in solution, this region is responsible for the Atg7 interaction which is an E1-like enzyme. | → the first region has 80 residues and has a random coil structure in solution, this region is responsible for the Atg7 interaction which is an E1-like enzyme. | ||
| Line 26: | Line 28: | ||
Moreover some researches indicates that the catalytic cysteine of Atg3 is a possible binding site for a phosphate of phosphatidylethanolamine. | Moreover some researches indicates that the catalytic cysteine of Atg3 is a possible binding site for a phosphate of phosphatidylethanolamine. | ||
===Atg8 protein=== | ===Atg8 protein=== | ||
| + | ---- | ||
| + | |||
Atg8 is a protein of 117 aminoacids with a molecular wieght of 13,6kDa. This molecule is composed of 5- stranded β-sheet. Those β-sheet are enclosed by two α-helices on each sides. This conformation leaves accessible a conserved GABARAP domain, this protein has been originally identified as a binding partner of a GABAA receptor subunit. [https://en.wikipedia.org/wiki/GABARAP GABARAP]. Even if the sequences between Atg8 and ubiquitin are not similars, the crystal structure reveals a conserved ubiquitine-like fold. Atg8 belongs to the ATG family but it differs from the other members of the family because the α2 helix-terminating proline 26 was substituted by a lysine. | Atg8 is a protein of 117 aminoacids with a molecular wieght of 13,6kDa. This molecule is composed of 5- stranded β-sheet. Those β-sheet are enclosed by two α-helices on each sides. This conformation leaves accessible a conserved GABARAP domain, this protein has been originally identified as a binding partner of a GABAA receptor subunit. [https://en.wikipedia.org/wiki/GABARAP GABARAP]. Even if the sequences between Atg8 and ubiquitin are not similars, the crystal structure reveals a conserved ubiquitine-like fold. Atg8 belongs to the ATG family but it differs from the other members of the family because the α2 helix-terminating proline 26 was substituted by a lysine. | ||
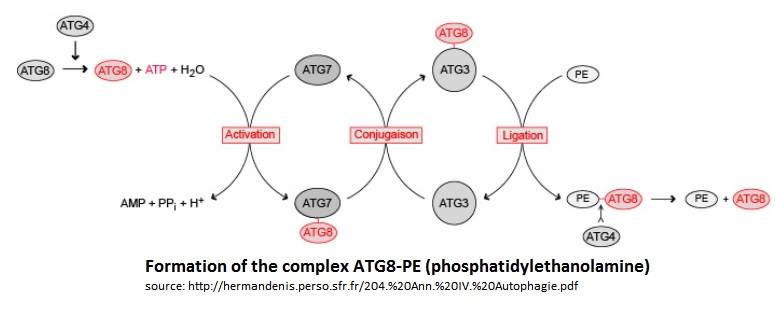
===Atg8/Atg3 Complex=== | ===Atg8/Atg3 Complex=== | ||
| + | ---- | ||
| - | + | Atg3 and Atg8 interact through a thioester bond between the Cys-288 of Atg3 and the C-terminal Glycine of Atg8. | |
| - | + | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
http://hermandenis.perso.sfr.fr/204.%20Ann.%20IV.%20Autophagie.pdf | http://hermandenis.perso.sfr.fr/204.%20Ann.%20IV.%20Autophagie.pdf | ||
Revision as of 19:47, 29 January 2016
| This Sandbox is Reserved from 15/12/2015, through 15/06/2016 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1120 through Sandbox Reserved 1159. |
To get started:
More help: Help:Editing |
Plasmodium falciparum Atg8 in complex with Plasmodium falciparum Atg3 peptide
| |||||||||||
References
http://hermandenis.perso.sfr.fr/204.%20Ann.%20IV.%20Autophagie.pdf