We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1126
From Proteopedia
(Difference between revisions)
| Line 10: | Line 10: | ||
It is encoded by the CBS gene located on chromosome 21 (at position 22.3). | It is encoded by the CBS gene located on chromosome 21 (at position 22.3). | ||
| - | == Structure | + | == Structure == |
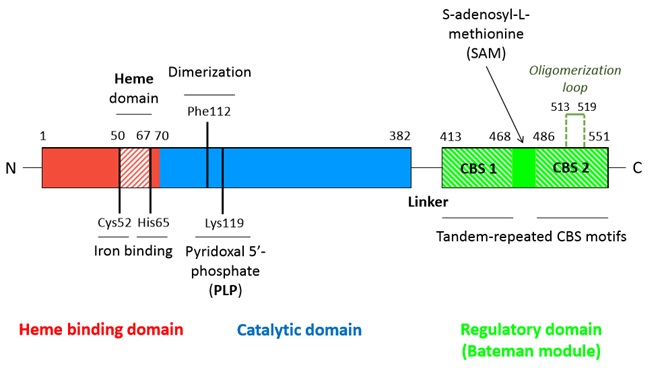
A CBS monomer is natively a 63 kDa protein made of 551 amino-acids and each of them binds two cofactors (the iron heme and the pyridoxal phosphate), as well as two substrates (homocysteine and serine). Hence, a CBS monomer contains (from N-terminal to C-terminal): <br/> | A CBS monomer is natively a 63 kDa protein made of 551 amino-acids and each of them binds two cofactors (the iron heme and the pyridoxal phosphate), as well as two substrates (homocysteine and serine). Hence, a CBS monomer contains (from N-terminal to C-terminal): <br/> | ||
| Line 51: | Line 51: | ||
== Allosteric regulation == | == Allosteric regulation == | ||
| - | + | ==== Autoinhibition (at the dimer scale) ==== | |
The Bateman modules natively prevent the access of the substrates to the catalytic site (PLP cavity) through: <br/> | The Bateman modules natively prevent the access of the substrates to the catalytic site (PLP cavity) through: <br/> | ||
| Line 59: | Line 59: | ||
| - | + | ==== Activation by S-adenosyl-L-methionine (SAM) ==== | |
| - | SAM is the allosteric activator of the CBS. It binds in a region located between the CBS1 and CBS2 domains of the Bateman module which is solvent-exposed and has less hefty hydrophobic residues. Moreover, this region shapes a hydrophobic cage able to host the adenine ring. Moreover threonine (T535) and aspartate (D538) help stabilizing the ribose through hydrogen bounds and polar interactions. | + | *SAM is the allosteric activator of the CBS. It binds in a region located between the CBS1 and CBS2 domains of the Bateman module which is solvent-exposed and has less hefty hydrophobic residues. Moreover, this region shapes a hydrophobic cage able to host the adenine ring. Moreover threonine (T535) and aspartate (D538) help stabilizing the ribose through hydrogen bounds and polar interactions. |
| - | Binding of SAM to the Bateman module destabilizes the interactions which sustain the tetramer structure and thus triggers the dissociation of the tetrameric structure into two dimers. | + | *Binding of SAM to the Bateman module destabilizes the interactions which sustain the tetramer structure and thus triggers the dissociation of the tetrameric structure into two dimers. |
| - | SAM fixation on the C-terminal regulatory domain entails the small rotation (or at least displacement) of the CBS1 and CBS2 of the Bateman module, thus distabilizing its interactions (hydrophobic interactions and hydrogen bounds) with the catalytic core of the other monomer. As a result, the Bateman module moves away from the catalytic core (this movement is all the more facilitated as the linker region is long enough and made of flexible residues). Loops 145-148, 171-174 and 191-202, previously involved in maintaining the close conformation through their interaction with the Bateman module, then relax and allow accessibility to the catalytic site (open conformation). SAM release leads to the return to the native inactive close conformation. | + | *SAM fixation on the C-terminal regulatory domain entails the small rotation (or at least displacement) of the CBS1 and CBS2 of the Bateman module, thus distabilizing its interactions (hydrophobic interactions and hydrogen bounds) with the catalytic core of the other monomer. As a result, the Bateman module moves away from the catalytic core (this movement is all the more facilitated as the linker region is long enough and made of flexible residues). Loops 145-148, 171-174 and 191-202, previously involved in maintaining the close conformation through their interaction with the Bateman module, then relax and allow accessibility to the catalytic site (open conformation). SAM release leads to the return to the native inactive close conformation. |
Revision as of 21:44, 29 January 2016
| This Sandbox is Reserved from 15/12/2015, through 15/06/2016 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1120 through Sandbox Reserved 1159. |
To get started:
More help: Help:Editing |
Human cystathionine β-synthase (hCBS)
| |||||||||||