Sandbox Reserved 1131
From Proteopedia
| Line 1: | Line 1: | ||
<Structure load='2NMM' size='350' frame='true' align='right' caption='Human Phosphohistidine phosphatase 1 (Homotrimer)' scene='Insert optional scene name here' /> Human Phosphohistidine phosphatase 1 {{Sandbox_Reserved_ESBS_2015}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | <Structure load='2NMM' size='350' frame='true' align='right' caption='Human Phosphohistidine phosphatase 1 (Homotrimer)' scene='Insert optional scene name here' /> Human Phosphohistidine phosphatase 1 {{Sandbox_Reserved_ESBS_2015}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
| - | (Human) Phosphohistidine phosphatase 1 belongs to the Janus family, | + | (Human) Phosphohistidine phosphatase 1 belongs to the Janus family, it has 2 isoforms produced by alternative splicing, and 6 transcripts. |
It is encoded by the PHPT1 gene, located on the 9th chromosome. | It is encoded by the PHPT1 gene, located on the 9th chromosome. | ||
| Line 8: | Line 8: | ||
<Structure load='2ai6' size='350' frame='true' align='right' caption='Solution structure of a Human Phosphohistidine Phosphatase 1 monomer' scene='Insert optional scene name here' /> | <Structure load='2ai6' size='350' frame='true' align='right' caption='Solution structure of a Human Phosphohistidine Phosphatase 1 monomer' scene='Insert optional scene name here' /> | ||
===Generality=== | ===Generality=== | ||
| - | Phosphohistidine phosphatase 1 is a 14kDa homotrimeric protein, | + | Phosphohistidine phosphatase 1 is a 14kDa homotrimeric protein, whose monomers contain all 125 amino acids. |
Furthermore, one monomer contains 4 α helices, 6 β strands and 2 turns. | Furthermore, one monomer contains 4 α helices, 6 β strands and 2 turns. | ||
| + | |||
===Domains=== | ===Domains=== | ||
| - | PHPT1 contains acetylation site and a N-acetylalanine site. | + | PHPT1 contains an acetylation site and a N-acetylalanine site. |
There is also an Janus/Ocnus family region, characteristic. | There is also an Janus/Ocnus family region, characteristic. | ||
| Line 37: | Line 38: | ||
= Implication in biological fonctions and pathways = | = Implication in biological fonctions and pathways = | ||
| + | Histidine reversible phosphorylation plays important roles in several signal transductions and other cellular functions. | ||
| + | |||
| + | === Inhibition of epithelial Ca²⁺ channel TRPV5 === | ||
| + | This channel is activated by the kinase NDPK-B-, which phosphorylates his(711) in the C-terminal tail of TRPV5. PHPT1 inhibits the activity of TRPV5 by dephosphorylating the same residue, resulting in a decrease in Ca²⁺ flux. This mechanism plays a role in the regulation of Ca²⁺ reabsorption by the kidney. | ||
= Diseases and medical applications = | = Diseases and medical applications = | ||
Revision as of 17:15, 30 January 2016
|
| This Sandbox is Reserved from 15/12/2015, through 15/06/2016 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1120 through Sandbox Reserved 1159. |
To get started:
More help: Help:Editing |
(Human) Phosphohistidine phosphatase 1 belongs to the Janus family, it has 2 isoforms produced by alternative splicing, and 6 transcripts. It is encoded by the PHPT1 gene, located on the 9th chromosome.
Contents |
Structure
|
Generality
Phosphohistidine phosphatase 1 is a 14kDa homotrimeric protein, whose monomers contain all 125 amino acids. Furthermore, one monomer contains 4 α helices, 6 β strands and 2 turns.
Domains
PHPT1 contains an acetylation site and a N-acetylalanine site. There is also an Janus/Ocnus family region, characteristic.
It has one substrate binding site, and one proton acceptor active site.
Function
It is an hydrolase. This characteristic structure allows it to have many activities : phosphoprotein and phosphohistidine phosphatase, calcium channel inhibition, ion channel binding. It is located in the cytosol and in extracellular exosome.
Active site
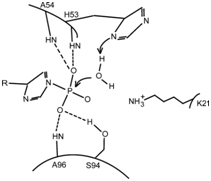
The active site is located between the beginning of helix α1 and loop L5. The catalytic residue is His(53), and the anchor sites of P(i) are the amine groups of His(53), Ala(54) and Ala(96) and the Hydroxy group of Ser(94), which form hydrogen bonds with it.
Ligands and binding
There are 6 SO4 lingands, called SO4 201 to 206. 2 lingand fix to each monomer (2ai6), inducing a conformational change of all the monomers (2hw4). Then, the lingands bind to each other, forming a trimer (2NMM). O4 S is a 96 Da molecule, with two negative charges. The four oxygens allow good fixation on the monomers, with hydrogen bounds.
Catalyic mechanism
The P(i) substrate binds to the active site thanks to the formation of four H-bonds. The imidazole ring of His(53) acts as a base to activate a water molecule, which will in turn do a nucleophilic attack on the substrate. The amine group of the residue Lys(21) can help stabilize the transition state.
Implication in biological fonctions and pathways
Histidine reversible phosphorylation plays important roles in several signal transductions and other cellular functions.
Inhibition of epithelial Ca²⁺ channel TRPV5
This channel is activated by the kinase NDPK-B-, which phosphorylates his(711) in the C-terminal tail of TRPV5. PHPT1 inhibits the activity of TRPV5 by dephosphorylating the same residue, resulting in a decrease in Ca²⁺ flux. This mechanism plays a role in the regulation of Ca²⁺ reabsorption by the kidney.
Diseases and medical applications
Structural highlights
</StructureSection>