Sandbox Reserved 1131
From Proteopedia
| Line 54: | Line 54: | ||
This dephosphorylation inhibits the activity of the KCa3.1 channel and decreases the Ca²⁺ afflux, negatively regulating human CD4 T cells. | This dephosphorylation inhibits the activity of the KCa3.1 channel and decreases the Ca²⁺ afflux, negatively regulating human CD4 T cells. | ||
| - | ===NB:dephosphorylation on a non-histidine residue === | + | ====NB:dephosphorylation on a non-histidine residue ==== |
A study has proved that PHPT1 dephosphorylate histone H1 and polylysine, which do not contain Histidine residue, meaning that the protin has a broader specificity than the one that we know yet. | A study has proved that PHPT1 dephosphorylate histone H1 and polylysine, which do not contain Histidine residue, meaning that the protin has a broader specificity than the one that we know yet. | ||
Revision as of 18:17, 30 January 2016
|
| This Sandbox is Reserved from 15/12/2015, through 15/06/2016 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1120 through Sandbox Reserved 1159. |
To get started:
More help: Help:Editing |
(Human) Phosphohistidine phosphatase 1 belongs to the Janus family, it has 2 isoforms produced by alternative splicing, and 6 transcripts. It is encoded by the PHPT1 gene, located on the 9th chromosome. This enzyme has been recently discovered so it has not been fully understood and characterized yet.
Contents |
Structure
|
Generality
Phosphohistidine phosphatase 1 is a 14kDa homotrimeric protein, whose monomers contain all 125 amino acids. Furthermore, one monomer contains 4 α helices, 6 β strands and 2 turns.
Domains
PHPT1 contains an acetylation site and a N-acetylalanine site. There is also an Janus/Ocnus family region, characteristic.
It has one substrate binding site, and one proton acceptor active site.
Function
It is an hydrolase. This characteristic structure allows it to have many activities : phosphoprotein and phosphohistidine phosphatase, calcium channel inhibition, ion channel binding. It is located in the cytosol and in extracellular exosome
Active site
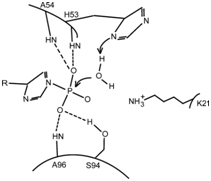
The active site is located between the beginning of helix α1 and loop L5. The catalytic residue is His(53), and the anchor sites of P(i) are the amine groups of His(53), Ala(54) and Ala(96) and the Hydroxy group of Ser(94), which form hydrogen bonds with it.
Ligands and binding
There are 6 SO4 lingands, called SO4 201 to 206. 2 lingand fix to each monomer (2ai6), inducing a conformational change of all the monomers (2hw4). Then, the lingands bind to each other, forming a trimer (2NMM). O4 S is a 96 Da molecule, with two negative charges. The four oxygens allow good fixation on the monomers, with hydrogen bounds.
Catalyic mechanism
The P(i) substrate binds to the active site thanks to the formation of four H-bonds. The imidazole ring of His(53) acts as a base to activate a water molecule, which will in turn do a nucleophilic attack on the substrate. The amine group of the residue Lys(21) can help stabilize the transition state.
Degradation of defective PHPT1
A possible degratation of PHPT1, thanks to quality control and cellular system self-guarding, can happen at two levels. A primary degradation of non-sens mutated PHPT1 mRNA is possible, at the RNA level. Then, the formed protein can be destroyed by the ptoteasome, if it is defective.
Implication in biological fonctions and pathways
Histidine reversible phosphorylation plays important roles in several signal transductions and other cellular functions.
Tissue expression
It is mainly expressed in the cytosol of cells located in the pancreas, the heart, and skeletal muscles.
Inhibition of epithelial Ca²⁺ channel TRPV5
This channel is activated by the kinase NDPK-B, which phosphorylates his(711) in the C-terminal tail of TRPV5. PHPT1 inhibits the activity of TRPV5 by dephosphorylating the same residue, resulting in a decrease in Ca²⁺ flux. This mechanism plays a role in the regulation of Ca²⁺ reabsorption by the kidney.
Inhibition of the K+ channel KCa3.1
PHPT1 dephosphorylates his(358) on KCa3.1, a Ca²⁺-dependant K+ channel that is activated by the phosphorylation on the same residue by NDPK-B. KCa3.1 helps maintain the negative membrane potential, and plays an important role in CD4 T cells signalling. This dephosphorylation inhibits the activity of the KCa3.1 channel and decreases the Ca²⁺ afflux, negatively regulating human CD4 T cells.
NB:dephosphorylation on a non-histidine residue
A study has proved that PHPT1 dephosphorylate histone H1 and polylysine, which do not contain Histidine residue, meaning that the protin has a broader specificity than the one that we know yet.
Diseases and medical applications
Lung Cancer
It has been proved that PHPT1 concentration is linked to lung cancer. Indeed, PHPT1 is associated with the carcinogenesis and metastasis of this cancer, it promotes cell migration and invasion. In cancerous cells, the expression of PHPT1 is almost two fold the one in in normal cells. Therefore, a therapy has been recently developped, aiming the inhibition or the silencing of PHPT1, and it is hoped to be benefic for lung cancer patient.