We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1127
From Proteopedia
(Difference between revisions)
| Line 47: | Line 47: | ||
==Positive regulation== | ==Positive regulation== | ||
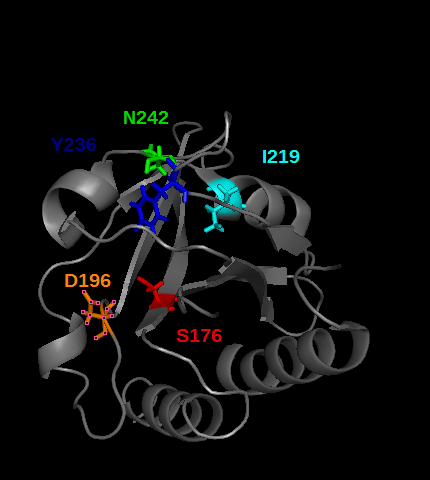
PDE5 contains several cGMP binding sites that do not have the same effect on its activity. These allosteric domains are GAF A and GAF B play a rôle in PDE5 activity. Especially GAF A(see the picture below) binds cGMP, by establishing hydrogens bonds between cGMP and the residues T,I, colored in the picture below. | PDE5 contains several cGMP binding sites that do not have the same effect on its activity. These allosteric domains are GAF A and GAF B play a rôle in PDE5 activity. Especially GAF A(see the picture below) binds cGMP, by establishing hydrogens bonds between cGMP and the residues T,I, colored in the picture below. | ||
| + | ** | ||
| + | ** | ||
| + | ** | ||
| + | ** | ||
[[Image:Free_GAF_A_domain_residues_that_binds_cGMP.png]] | [[Image:Free_GAF_A_domain_residues_that_binds_cGMP.png]] | ||
CGMP binding to GAF A trigegr an allosteric modification that lead to the seperation of the dimeric catalytic site of the enzyme, so that it opens cGMP access to the catalytic site. | CGMP binding to GAF A trigegr an allosteric modification that lead to the seperation of the dimeric catalytic site of the enzyme, so that it opens cGMP access to the catalytic site. | ||
Revision as of 19:00, 30 January 2016
| This Sandbox is Reserved from 15/12/2015, through 15/06/2016 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1120 through Sandbox Reserved 1159. |
To get started:
More help: Help:Editing |
Human PDE5
| |||||||||||