Sandbox Reserved 1127
From Proteopedia
| Line 49: | Line 49: | ||
==Allosteric regulation== | ==Allosteric regulation== | ||
| - | + | Positive regulation | |
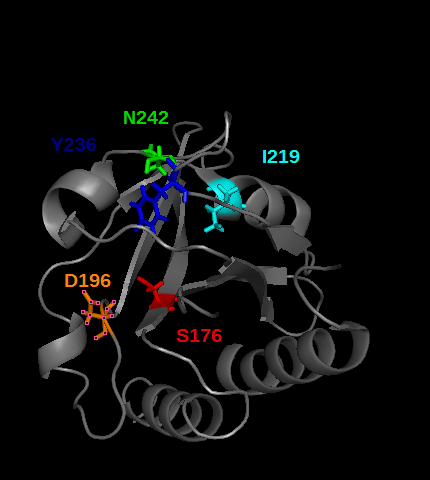
PDE5 contains several cGMP binding sites that do not have the same effect on its activity. These allosteric domains are GAF A and GAF B play a rôle in PDE5 activity. Especially GAF A(see the picture below) binds cGMP, by establishing hydrogens bonds between cGMP and the residues T,I, colored in the picture below. | PDE5 contains several cGMP binding sites that do not have the same effect on its activity. These allosteric domains are GAF A and GAF B play a rôle in PDE5 activity. Especially GAF A(see the picture below) binds cGMP, by establishing hydrogens bonds between cGMP and the residues T,I, colored in the picture below. | ||
| Line 56: | Line 56: | ||
| - | CGMP binding to GAF A | + | CGMP binding to GAF A trigger an allosteric modification that lead to the seperation of the dimeric catalytic site of the enzyme, so that it opens cGMP access to the catalytic site. |
This cGMP binding to allosteric sites increases the enzyme affinity to cGMP. | This cGMP binding to allosteric sites increases the enzyme affinity to cGMP. | ||
Plus,the phosphorylation the Ser92 lead to increase the catalytic activity of the enzyme. | Plus,the phosphorylation the Ser92 lead to increase the catalytic activity of the enzyme. | ||
| - | + | ||
| - | In large excess og cGMP, PKG cGMP, is sequestred by the allosteric sites and can no longer binds the catalytic site | + | Negative regulation |
| + | In large excess og cGMP, PKG cGMP, is sequestred by the allosteric sites and can no longer binds the catalytic site. An other includes both PKG and the myosine phosphatase. | ||
These allosteric processes can both explain activation of PDE5 but also the negative feedback of cGMP on the phosphodiesterase. | These allosteric processes can both explain activation of PDE5 but also the negative feedback of cGMP on the phosphodiesterase. | ||
Revision as of 19:30, 30 January 2016
| This Sandbox is Reserved from 15/12/2015, through 15/06/2016 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1120 through Sandbox Reserved 1159. |
To get started:
More help: Help:Editing |
Human PDE5
Contributors
DJAGO Fabiola, AL BADAWY Kays, CHOI Ji-Hyung
| |||||||||||
References
[1] [2] [3] [4] ["Mammalian Cyclic Nucleotide Phosphodiesterases: Molecular Mechanisms and Physiological Functions", SHARRON H. FRANCIS, MITSI A. BLOUNT, AND JACKIE D. CORBIN, Physiol Rev 91: 651–690, 2011; doi:10.1152/physrev.00030.2010.] [« Solution Structure of the cGMP Binding GAF Domain from Phosphodiesterase 5 », Clemens C. Heikaus, Joseph R.Stout, Monica R. Sekharan, Catherine M. Eakin, Ponni Rajagopal, Peter S. Brzovic, Joseph A,J Biol Chem. 2008 Aug 15; 283(33): 22749–22759.] [« Regulation of cGMP-specific Phosphodiesterase(PDE5) Phosphorylation in Smooth Muscle Cells », Sergei D.Rybalkin, Irina G. Rybalkina, Robert Feil, Franz Hoffman and Joseph A.Beavo,February 1, 2002 The Journal of Biological Chemistry, 277,3310-3317.] [« Allosteric sites of phosphodiesterase-5 (PDE5) A potential role in negative feedback regulation of cGMP signaling in corpus cavernosum »,Venkatesh K. Gopal, Sharron H. Francis and Jackie D. Corbin ,Eur. J. Biochem. 268, 3304±3312 (2001)]