We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1126
From Proteopedia
(Difference between revisions)
| Line 38: | Line 38: | ||
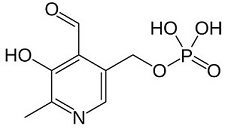
<center>[[Image:Pyridoxal phosphate.jpg]] © Wikipedia</center> <br/> | <center>[[Image:Pyridoxal phosphate.jpg]] © Wikipedia</center> <br/> | ||
| - | Pyridoxal phosphate (PLP) is the active form of the vitamin B6 (= pyridoxal, pyridoxamine and pyridoxine). | + | Pyridoxal phosphate (PLP) is the active form of the vitamin B6 (= pyridoxal, pyridoxamine and pyridoxine). It is the coenzyme of a lot of enzymatic reactions (transamination, decarboxylation, deamination, etc). |
'''Action mechanism''': the aldehyde group of PLP forms a Schiff-base linkage with the epsilon-amino group of an active site lysine residue on the enzyme. Then, the alpha-amino group of the substrate displaces the epsilon-amino group of the active-site lysine residue. Finally, the result is the formation of a new aldimine with the substrate, which is the intermediate for all reactions which are catalysed by PLP. | '''Action mechanism''': the aldehyde group of PLP forms a Schiff-base linkage with the epsilon-amino group of an active site lysine residue on the enzyme. Then, the alpha-amino group of the substrate displaces the epsilon-amino group of the active-site lysine residue. Finally, the result is the formation of a new aldimine with the substrate, which is the intermediate for all reactions which are catalysed by PLP. | ||
| Line 44: | Line 44: | ||
Enzymes which depend on PLP are mainly involved in the synthesis of amino acids and amino acid-derived metabolites. Moreover, they can also be found in the pathway of amino sugars and in the synthesis/catabolism of neurotransmitters. | Enzymes which depend on PLP are mainly involved in the synthesis of amino acids and amino acid-derived metabolites. Moreover, they can also be found in the pathway of amino sugars and in the synthesis/catabolism of neurotransmitters. | ||
| - | An inadequate level of PLP in the brain can be | + | An inadequate level of PLP in the brain can be responsible for neurological dysfunction, for example epilepsy. |
Revision as of 20:20, 30 January 2016
| This Sandbox is Reserved from 15/12/2015, through 15/06/2016 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1120 through Sandbox Reserved 1159. |
To get started:
More help: Help:Editing |
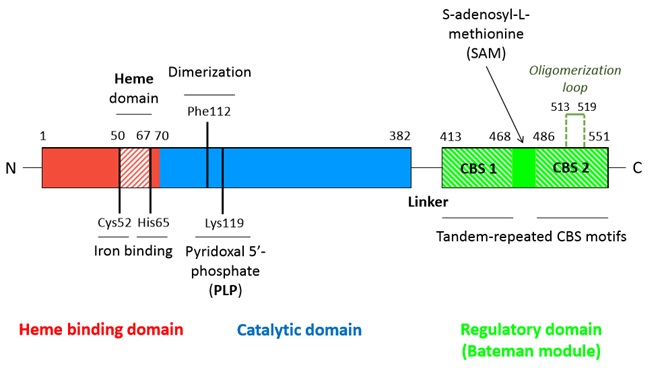
Human cystathionine β-synthase (hCBS)
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 Meier M, Janosik M, Kery V, Kraus JP, Burkhard P. Structure of human cystathionine beta-synthase: a unique pyridoxal 5'-phosphate-dependent heme protein. EMBO J. 2001 Aug 1;20(15):3910-6. PMID:11483494 doi:http://dx.doi.org/10.1093/emboj/20.15.3910
- ↑ Regnier V, Billard JM, Gupta S, Potier B, Woerner S, Paly E, Ledru A, David S, Luilier S, Bizot JC, Vacano G, Kraus JP, Patterson D, Kruger WD, Delabar JM, London J. Brain phenotype of transgenic mice overexpressing cystathionine beta-synthase. PLoS One. 2012;7(1):e29056. doi: 10.1371/journal.pone.0029056. Epub 2012 Jan 12. PMID:22253703 doi:http://dx.doi.org/10.1371/journal.pone.0029056
- ↑ McCorvie TJ, Kopec J, Hyung SJ, Fitzpatrick F, Feng X, Termine D, Strain-Damerell C, Vollmar M, Fleming J, Janz JM, Bulawa C, Yue WW. Inter-Domain Communication Of Human Cystathionine Beta Synthase: Structural Basis Of S-Adenosyl-L-Methionine Activation. J Biol Chem. 2014 Oct 21. pii: jbc.M114.610782. PMID:25336647 doi:http://dx.doi.org/10.1074/jbc.M114.610782
- ↑ 4.0 4.1 4.2 Ereno-Orbea J, Majtan T, Oyenarte I, Kraus JP, Martinez-Cruz LA. Structural basis of regulation and oligomerization of human cystathionine beta-synthase, the central enzyme of transsulfuration. Proc Natl Acad Sci U S A. 2013 Sep 16. PMID:24043838 doi:10.1073/pnas.1313683110
- ↑ Miles EW, Kraus JP. Cystathionine beta-synthase: structure, function, regulation, and location of homocystinuria-causing mutations. J Biol Chem. 2004 Jul 16;279(29):29871-4. Epub 2004 Apr 15. PMID:15087459 doi:http://dx.doi.org/10.1074/jbc.R400005200
- ↑ 6.0 6.1 Ereno-Orbea J, Majtan T, Oyenarte I, Kraus JP, Martinez-Cruz LA. Structural insight into the molecular mechanism of allosteric activation of human cystathionine beta-synthase by S-adenosylmethionine. Proc Natl Acad Sci U S A. 2014 Sep 16;111(37):E3845-52. doi:, 10.1073/pnas.1414545111. Epub 2014 Sep 2. PMID:25197074 doi:http://dx.doi.org/10.1073/pnas.1414545111