We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1126
From Proteopedia
(Difference between revisions)
| Line 86: | Line 86: | ||
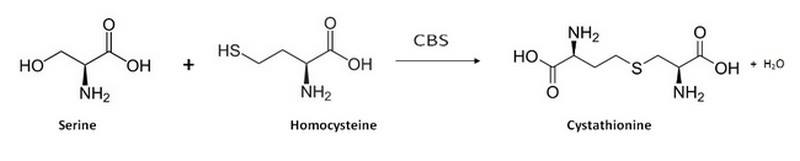
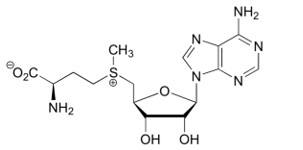
On the contrary, a '''reduced activity of CBS''' leads to '''homocystinuria'''. This disorder is inherited in an autosomal recessive pattern and is caused by loss-of-function mutations in the hCBS gene. Those mutations interfere with the activation of CBS. <br/> Thus, Homocystinuria is characterized by high plasma levels of the toxic amino acid homocysteine and infants who develop this disease may have difficulties to grow and gain weight accompanied by mental retardation. | On the contrary, a '''reduced activity of CBS''' leads to '''homocystinuria'''. This disorder is inherited in an autosomal recessive pattern and is caused by loss-of-function mutations in the hCBS gene. Those mutations interfere with the activation of CBS. <br/> Thus, Homocystinuria is characterized by high plasma levels of the toxic amino acid homocysteine and infants who develop this disease may have difficulties to grow and gain weight accompanied by mental retardation. | ||
| - | |||
| - | == Relevance == | ||
| - | This is the <scene name='71/719867/Scene_1/1'>active site</scene> of the chain A of the protein. | ||
| - | This is the <scene name='71/719867/Scene_2/1'>C-terminal domain</scene> of the chain A of the protein. | ||
| - | This is the <scene name='71/719867/Scene_3/1'>loop involved in the tetramer formation</scene>. | ||
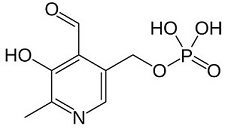
| - | This is the <scene name='71/719867/Scene_4/1'>PLP</scene> of the protein. | ||
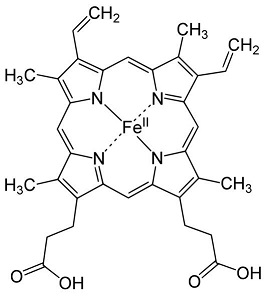
| - | This is the <scene name='71/719867/Scene_4/2'>HEME</scene> of the protein. | ||
| - | This is the <scene name='71/719867/Scene_5/1'>loop controlling the catalytic site</scene>. | ||
| - | This is the <scene name='71/719867/Scene_5/2'>Loop 191-202</scene> of the protein. | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 21:14, 30 January 2016
| This Sandbox is Reserved from 15/12/2015, through 15/06/2016 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1120 through Sandbox Reserved 1159. |
To get started:
More help: Help:Editing |
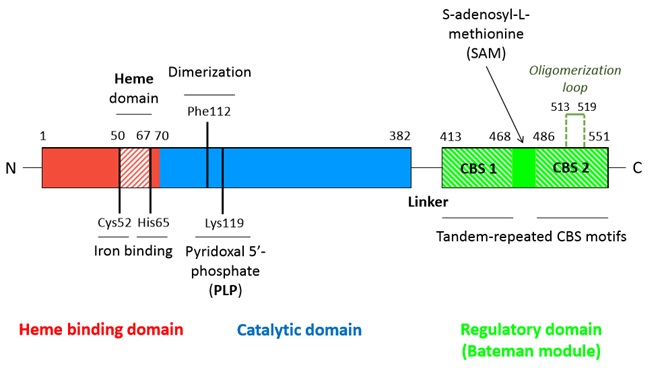
Human cystathionine β-synthase (hCBS)
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 Meier M, Janosik M, Kery V, Kraus JP, Burkhard P. Structure of human cystathionine beta-synthase: a unique pyridoxal 5'-phosphate-dependent heme protein. EMBO J. 2001 Aug 1;20(15):3910-6. PMID:11483494 doi:http://dx.doi.org/10.1093/emboj/20.15.3910
- ↑ Regnier V, Billard JM, Gupta S, Potier B, Woerner S, Paly E, Ledru A, David S, Luilier S, Bizot JC, Vacano G, Kraus JP, Patterson D, Kruger WD, Delabar JM, London J. Brain phenotype of transgenic mice overexpressing cystathionine beta-synthase. PLoS One. 2012;7(1):e29056. doi: 10.1371/journal.pone.0029056. Epub 2012 Jan 12. PMID:22253703 doi:http://dx.doi.org/10.1371/journal.pone.0029056
- ↑ McCorvie TJ, Kopec J, Hyung SJ, Fitzpatrick F, Feng X, Termine D, Strain-Damerell C, Vollmar M, Fleming J, Janz JM, Bulawa C, Yue WW. Inter-Domain Communication Of Human Cystathionine Beta Synthase: Structural Basis Of S-Adenosyl-L-Methionine Activation. J Biol Chem. 2014 Oct 21. pii: jbc.M114.610782. PMID:25336647 doi:http://dx.doi.org/10.1074/jbc.M114.610782
- ↑ 4.0 4.1 4.2 Ereno-Orbea J, Majtan T, Oyenarte I, Kraus JP, Martinez-Cruz LA. Structural basis of regulation and oligomerization of human cystathionine beta-synthase, the central enzyme of transsulfuration. Proc Natl Acad Sci U S A. 2013 Sep 16. PMID:24043838 doi:10.1073/pnas.1313683110
- ↑ Miles EW, Kraus JP. Cystathionine beta-synthase: structure, function, regulation, and location of homocystinuria-causing mutations. J Biol Chem. 2004 Jul 16;279(29):29871-4. Epub 2004 Apr 15. PMID:15087459 doi:http://dx.doi.org/10.1074/jbc.R400005200
- ↑ http://www.ebi.ac.uk/interpro/entry/IPR004625
- ↑ https://pubchem.ncbi.nlm.nih.gov/compound/pyridoxal_phosphate#section=Top
- ↑ 8.0 8.1 Ereno-Orbea J, Majtan T, Oyenarte I, Kraus JP, Martinez-Cruz LA. Structural insight into the molecular mechanism of allosteric activation of human cystathionine beta-synthase by S-adenosylmethionine. Proc Natl Acad Sci U S A. 2014 Sep 16;111(37):E3845-52. doi:, 10.1073/pnas.1414545111. Epub 2014 Sep 2. PMID:25197074 doi:http://dx.doi.org/10.1073/pnas.1414545111