Sandbox Reserved 1127
From Proteopedia
| Line 10: | Line 10: | ||
== Function == | == Function == | ||
| - | PDE5 is 5th isoform of the 12 known phosphodiesterases. This enzyme family is responsible for cyclic AMP and cyclic GMP(cGMP) hydrolysis into AMP and GMP. :PE5 specifically catalyzes the hydrolysis of cGMP into 5'GMP. cGMP is an usual second messager in cell signalling pathways, like NO synthesis. By | + | PDE5 is 5th isoform of the 12 known phosphodiesterases. This enzyme family is responsible for cyclic AMP and cyclic GMP(cGMP) hydrolysis into AMP and GMP. :PE5 specifically catalyzes the hydrolysis of cGMP into 5'GMP. cGMP is an usual second messager in cell signalling pathways, like NO synthesis. By degradating such nucleotide,this phosphodiesterase of class I regulates nucletotic concentration of the cell, through an allosteric regulation system. |
PDE5 function affects the smooth muscles, blood vessels or penis, uterus and intestines. | PDE5 function affects the smooth muscles, blood vessels or penis, uterus and intestines. | ||
| Line 23: | Line 23: | ||
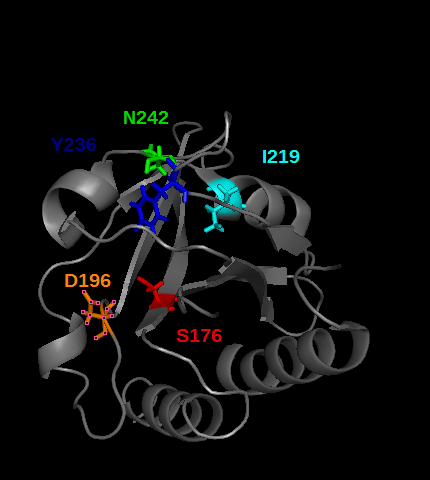
**Catalytic domain (588-853 amino acids) in Ctermini involved in cGMP hydrolysis. | **Catalytic domain (588-853 amino acids) in Ctermini involved in cGMP hydrolysis. | ||
The full structure of PDE5 has not been crystallized contrary to this of isolated domains of the protein. | The full structure of PDE5 has not been crystallized contrary to this of isolated domains of the protein. | ||
| - | GAFA and GAFB are homologous domains and allosteric binding | + | GAFA and GAFB are homologous domains and allosteric binding sites of cGMP. |
| - | The <scene name='71/719868/Structure/1'>secondary structure</scene> of the catalytic domain allows to see that this domain is mostly constituted by <span style="color:deeppink;background-color:black;font-weight:bold;">alpha helix</span> and <span style="color:darkmagenta;background-color:black;font-weight:bold;">turns</span>. | + | The <scene name='71/719868/Structure/1'>secondary structure</scene> of the catalytic domain allows us to see that this domain is mostly constituted by <span style="color:deeppink;background-color:black;font-weight:bold;">alpha helix</span> and <span style="color:darkmagenta;background-color:black;font-weight:bold;">turns</span>. |
==Catalytic activity== | ==Catalytic activity== | ||
| Line 57: | Line 57: | ||
CGMP binding to GAF A trigger an allosteric modification that lead to the seperation of the dimeric catalytic site of the enzyme, so that it opens cGMP access to the catalytic site. | CGMP binding to GAF A trigger an allosteric modification that lead to the seperation of the dimeric catalytic site of the enzyme, so that it opens cGMP access to the catalytic site. | ||
This cGMP binding to allosteric sites increases the enzyme affinity to cGMP. | This cGMP binding to allosteric sites increases the enzyme affinity to cGMP. | ||
| - | Plus,the phosphorylation the Ser92 | + | Plus,the phosphorylation the Ser92 by the PKG kinase leads to increase the catalytic activity of the enzyme. |
===Negative regulation=== | ===Negative regulation=== | ||
| - | In large excess og cGMP, | + | In large excess og cGMP, cGMP is sequestred by the allosteric sites and can no longer binds the catalytic site. An other model including both PKG and the myosine phosphatase shows that PKG realises an inhibitive phosphorylation of PDE5. In this way, the cell can regulate GMP concentration by a negative feedback. |
| - | These allosteric processes can both explain activation of PDE5 but also the negative feedback of cGMP on the phosphodiesterase. | ||
| Line 73: | Line 72: | ||
[http://www.ebi.ac.uk/interpro/sequencesearch/iprscan5-S20160124-100328-0067-2702087-es] | [http://www.ebi.ac.uk/interpro/sequencesearch/iprscan5-S20160124-100328-0067-2702087-es] | ||
["Mammalian Cyclic Nucleotide Phosphodiesterases: Molecular Mechanisms and Physiological Functions", SHARRON H. FRANCIS, MITSI A. BLOUNT, AND JACKIE D. CORBIN, Physiol Rev 91: 651–690, 2011; doi:10.1152/physrev.00030.2010.] | ["Mammalian Cyclic Nucleotide Phosphodiesterases: Molecular Mechanisms and Physiological Functions", SHARRON H. FRANCIS, MITSI A. BLOUNT, AND JACKIE D. CORBIN, Physiol Rev 91: 651–690, 2011; doi:10.1152/physrev.00030.2010.] | ||
| + | ["Phosphodiesterase-5 Gln817 Is Critical for cGMP, Vardenafil, or Sildenafil Affinity, its orientation impacts cGMP but not cAMP affinity, 2005 Published, JBC Papers in Press, 2006, Roya Zoraghi, Jackie D. Corbin, and Sharron H. Francis] | ||
[« Solution Structure of the cGMP Binding GAF Domain from Phosphodiesterase 5 », Clemens C. Heikaus, Joseph R.Stout, Monica R. Sekharan, Catherine M. Eakin, Ponni Rajagopal, Peter S. Brzovic, Joseph A,J Biol Chem. 2008 Aug 15; 283(33): 22749–22759.] | [« Solution Structure of the cGMP Binding GAF Domain from Phosphodiesterase 5 », Clemens C. Heikaus, Joseph R.Stout, Monica R. Sekharan, Catherine M. Eakin, Ponni Rajagopal, Peter S. Brzovic, Joseph A,J Biol Chem. 2008 Aug 15; 283(33): 22749–22759.] | ||
[« Regulation of cGMP-specific Phosphodiesterase(PDE5) Phosphorylation in Smooth Muscle Cells », Sergei D.Rybalkin, Irina G. Rybalkina, Robert Feil, Franz Hoffman and Joseph A.Beavo,February 1, 2002 The Journal of Biological Chemistry, 277,3310-3317.] | [« Regulation of cGMP-specific Phosphodiesterase(PDE5) Phosphorylation in Smooth Muscle Cells », Sergei D.Rybalkin, Irina G. Rybalkina, Robert Feil, Franz Hoffman and Joseph A.Beavo,February 1, 2002 The Journal of Biological Chemistry, 277,3310-3317.] | ||
Revision as of 06:33, 31 January 2016
| This Sandbox is Reserved from 15/12/2015, through 15/06/2016 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1120 through Sandbox Reserved 1159. |
To get started:
More help: Help:Editing |
Human PDE5
Contributors
DJAGO Fabiola, AL BADAWY Kays, CHOI Ji-Hyung
| |||||||||||
References
[1] [2] [3] [4] ["Mammalian Cyclic Nucleotide Phosphodiesterases: Molecular Mechanisms and Physiological Functions", SHARRON H. FRANCIS, MITSI A. BLOUNT, AND JACKIE D. CORBIN, Physiol Rev 91: 651–690, 2011; doi:10.1152/physrev.00030.2010.] ["Phosphodiesterase-5 Gln817 Is Critical for cGMP, Vardenafil, or Sildenafil Affinity, its orientation impacts cGMP but not cAMP affinity, 2005 Published, JBC Papers in Press, 2006, Roya Zoraghi, Jackie D. Corbin, and Sharron H. Francis] [« Solution Structure of the cGMP Binding GAF Domain from Phosphodiesterase 5 », Clemens C. Heikaus, Joseph R.Stout, Monica R. Sekharan, Catherine M. Eakin, Ponni Rajagopal, Peter S. Brzovic, Joseph A,J Biol Chem. 2008 Aug 15; 283(33): 22749–22759.] [« Regulation of cGMP-specific Phosphodiesterase(PDE5) Phosphorylation in Smooth Muscle Cells », Sergei D.Rybalkin, Irina G. Rybalkina, Robert Feil, Franz Hoffman and Joseph A.Beavo,February 1, 2002 The Journal of Biological Chemistry, 277,3310-3317.] [« Allosteric sites of phosphodiesterase-5 (PDE5) A potential role in negative feedback regulation of cGMP signaling in corpus cavernosum »,Venkatesh K. Gopal, Sharron H. Francis and Jackie D. Corbin ,Eur. J. Biochem. 268, 3304±3312 (2001)]