We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1127
From Proteopedia
(Difference between revisions)
| Line 49: | Line 49: | ||
==Allosteric regulation== | ==Allosteric regulation== | ||
===Positive regulation=== | ===Positive regulation=== | ||
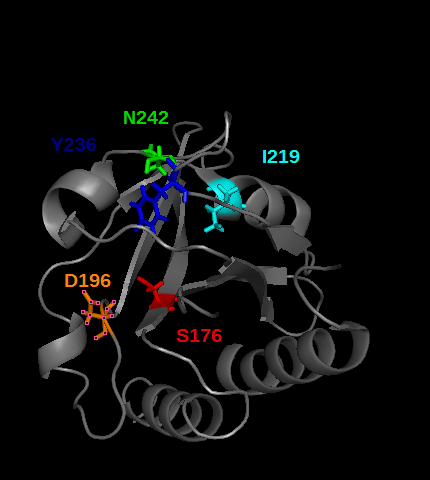
| - | PDE5 contains several cGMP binding sites that do not have the same effect on its activity. These allosteric domains are GAF A and GAF B play a role in PDE5 activity. Especially GAF A(see the picture below) binds cGMP, by establishing hydrogens bonds between cGMP and the residues T,I, colored in the picture below | + | PDE5 contains several cGMP binding sites that do not have the same effect on its activity. These allosteric domains are GAF A and GAF B play a role in PDE5 activity. Especially GAF A(see the picture below) binds cGMP, by establishing hydrogens bonds between cGMP and the residues T,I,<ref>DOI:10.1074/jbc.M801577200</ref> colored in the picture below |
| Line 57: | Line 57: | ||
cGMP binding to GAF A triggers an allosteric modification that lead to the seperation of the dimeric catalytic site of the enzyme, so that it free cGMP access to the catalytic site. | cGMP binding to GAF A triggers an allosteric modification that lead to the seperation of the dimeric catalytic site of the enzyme, so that it free cGMP access to the catalytic site. | ||
This cGMP binding to allosteric sites increases the enzyme affinity to cGMP. | This cGMP binding to allosteric sites increases the enzyme affinity to cGMP. | ||
| - | Plus,the phosphorylation the Ser92 by the PKG kinase leads to increase the catalytic activity of the enzyme. | + | Plus,the phosphorylation the Ser92 by the PKG kinase<ref>DOI:10.1093/emboj/cdg051</ref> leads to increase the catalytic activity of the enzyme. |
===Negative regulation=== | ===Negative regulation=== | ||
| Line 68: | Line 68: | ||
== References == | == References == | ||
<ref group="xtra">PMID:16407275</ref> | <ref group="xtra">PMID:16407275</ref> | ||
| + | <ref group="xtra">DOI:10.1093/emboj/cdg051</ref> | ||
| + | <ref group="xtra">DOI:10.1074/jbc.M801577200</ref> | ||
[http://www.phosphosite.org/proteinAction?id=1026&showAllSites=true] | [http://www.phosphosite.org/proteinAction?id=1026&showAllSites=true] | ||
[http://www.rcsb.org/pdb/explore/explore.do?pdbId=1TBF] | [http://www.rcsb.org/pdb/explore/explore.do?pdbId=1TBF] | ||
[http://www.uniprot.org/uniprot/O76074#structure] | [http://www.uniprot.org/uniprot/O76074#structure] | ||
[http://www.ebi.ac.uk/interpro/sequencesearch/iprscan5-S20160124-100328-0067-2702087-es] | [http://www.ebi.ac.uk/interpro/sequencesearch/iprscan5-S20160124-100328-0067-2702087-es] | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
<references/> | <references/> | ||
Revision as of 07:11, 31 January 2016
| This Sandbox is Reserved from 15/12/2015, through 15/06/2016 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1120 through Sandbox Reserved 1159. |
To get started:
More help: Help:Editing |
Human PDE5
Contributors
DJAGO Fabiola, AL BADAWY Kays, CHOI Ji-Hyung
| |||||||||||
References
- ↑ Zoraghi R, Corbin JD, Francis SH. Phosphodiesterase-5 Gln817 is critical for cGMP, vardenafil, or sildenafil affinity: its orientation impacts cGMP but not cAMP affinity. J Biol Chem. 2006 Mar 3;281(9):5553-8. Epub 2006 Jan 5. PMID:16407275 doi:http://dx.doi.org/10.1074/jbc.M510372200
- ↑ Heikaus CC, Stout JR, Sekharan MR, Eakin CM, Rajagopal P, Brzovic PS, Beavo JA, Klevit RE. Solution structure of the cGMP binding GAF domain from phosphodiesterase 5: insights into nucleotide specificity, dimerization, and cGMP-dependent conformational change. J Biol Chem. 2008 Aug 15;283(33):22749-59. Epub 2008 Jun 4. PMID:18534985 doi:10.1074/jbc.M801577200
- ↑ Rybalkin SD, Rybalkina IG, Shimizu-Albergine M, Tang XB, Beavo JA. PDE5 is converted to an activated state upon cGMP binding to the GAF A domain. EMBO J. 2003 Feb 3;22(3):469-78. PMID:12554648 doi:http://dx.doi.org/10.1093/emboj/cdg051