Pesticidal crystal protein
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
== Cry Proteins == | == Cry Proteins == | ||
<StructureSection load='1CIY' size='350' side='right' caption='Structure of cry protein Cry1Aa(PDB entry [[1ciy]])' scene=''> | <StructureSection load='1CIY' size='350' side='right' caption='Structure of cry protein Cry1Aa(PDB entry [[1ciy]])' scene=''> | ||
| - | Cry proteins are endotoxins produced by ''Bacillus thuringiensis'', and form crystal structures (thus the name "cry" proteins, short for crystal). Cry toxins have specific activities against insect species of the orders ''Lepidoptera'' (moths and butterflies), ''Diptera'' (flies and mosquitoes), ''Coleoptera'' (beetles), ''Hymenoptera'' (wasps, bees, ants and sawflies) and nematodes. When insects ingest toxin crystals, the alkaline pH of their digestive tract denatures the insoluble crystals, making them soluble and thus amenable to being cut with proteases found in the insect gut, which liberate the cry toxin from the crystal. The Cry toxin is then inserted into the insect gut cell membrane, paralyzing the digestive tract and forming a pore. The insect stops eating and starves to death; live Bt bacteria may also colonize the insect which can contribute to death. | + | '''Cry proteins''' are endotoxins produced by ''Bacillus thuringiensis'', and form crystal structures (thus the name "cry" proteins, short for crystal). Cry toxins have specific activities against insect species of the orders ''Lepidoptera'' (moths and butterflies), ''Diptera'' (flies and mosquitoes), ''Coleoptera'' (beetles), ''Hymenoptera'' (wasps, bees, ants and sawflies) and nematodes. When insects ingest toxin crystals, the alkaline pH of their digestive tract denatures the insoluble crystals, making them soluble and thus amenable to being cut with proteases found in the insect gut, which liberate the cry toxin from the crystal. The Cry toxin is then inserted into the insect gut cell membrane, paralyzing the digestive tract and forming a pore. The insect stops eating and starves to death; live Bt bacteria may also colonize the insect which can contribute to death. |
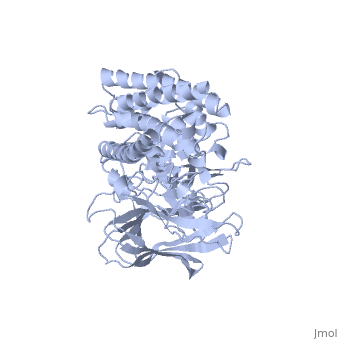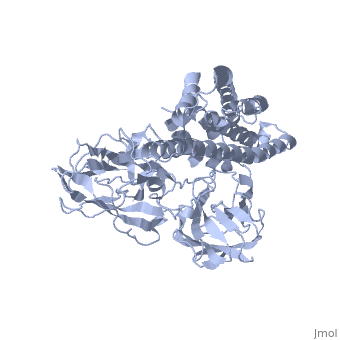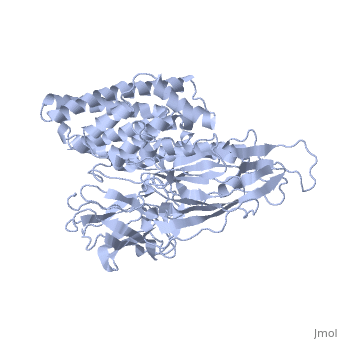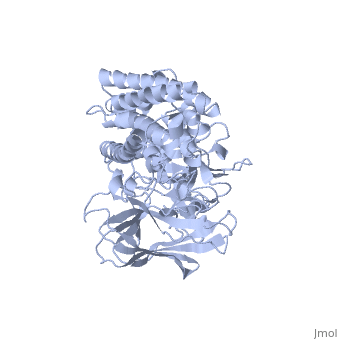
The cry protein has <scene name='57/570587/Three_domains/1'>three domains</scene>: an alpha helical N terminal domain, domain I; a Greek key beta sheet domain III; and a "jelly roll" antiparallel b-sandwich domain III. The core of the protein is the most highly <scene name='57/570587/Conserved_regions/1'>conserved</scene>, with Helix 5 of Domain I and the areas of contact between the domains being the most highly conserved (purple). | The cry protein has <scene name='57/570587/Three_domains/1'>three domains</scene>: an alpha helical N terminal domain, domain I; a Greek key beta sheet domain III; and a "jelly roll" antiparallel b-sandwich domain III. The core of the protein is the most highly <scene name='57/570587/Conserved_regions/1'>conserved</scene>, with Helix 5 of Domain I and the areas of contact between the domains being the most highly conserved (purple). | ||
Revision as of 10:06, 1 February 2016
Cry Proteins
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Ann Taylor, Alexander Berchansky, Jaime Prilusky