This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Prion
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
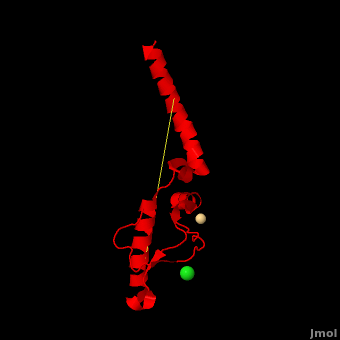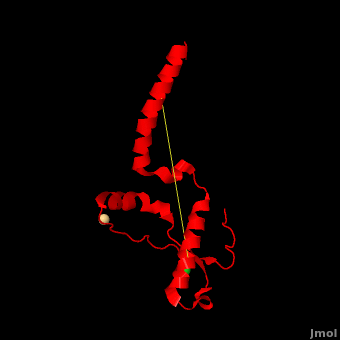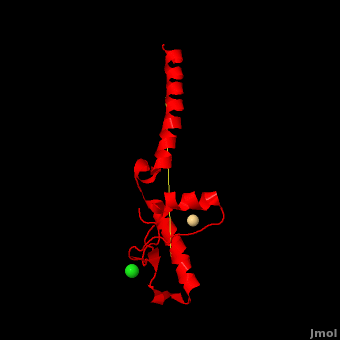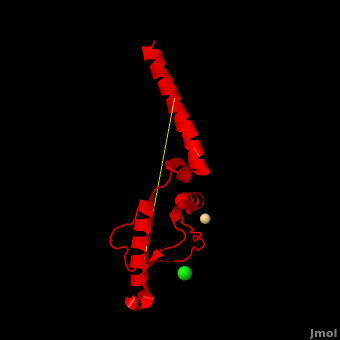
'''Prion''' (PrP) is a protein which becomes infectious upon undergoing conformation change to an amyloid form, which is self-propagating and becomes resistant to protease degradation. The fungus ''Podospora anserine'' has a prion-like protein HET-S which undergoes a conformation change to amyloid form which prevents its colony from merging with non-compatible colonies. Yeast prion proteins are Sup35 and Ure2. The images at the left and at the right correspond to one representative prion, ''i.e.'' the crystal structure of human prion ([[3haf]]). For more details see<br /> | '''Prion''' (PrP) is a protein which becomes infectious upon undergoing conformation change to an amyloid form, which is self-propagating and becomes resistant to protease degradation. The fungus ''Podospora anserine'' has a prion-like protein HET-S which undergoes a conformation change to amyloid form which prevents its colony from merging with non-compatible colonies. Yeast prion proteins are Sup35 and Ure2. The images at the left and at the right correspond to one representative prion, ''i.e.'' the crystal structure of human prion ([[3haf]]). For more details see<br /> | ||
*[[Prion protein]]<br /> | *[[Prion protein]]<br /> | ||
| - | *[[Human Prion Protein Dimer]] | + | *[[Human Prion Protein Dimer]]<br /> |
| + | *[[Doppel]],<br /> | ||
Click here to see <scene name='Prion/Cv/2'>example of prion unfolding</scene> (morph was taken from [http://molmovdb.org/cgi-bin/movie.cgi Gallery of Morphs] of the [http://molmovdb.org Yale Morph Server]). | Click here to see <scene name='Prion/Cv/2'>example of prion unfolding</scene> (morph was taken from [http://molmovdb.org/cgi-bin/movie.cgi Gallery of Morphs] of the [http://molmovdb.org Yale Morph Server]). | ||
Revision as of 07:49, 2 February 2016
| |||||||||||
3D structures of prion
Updated on 02-February-2016
- ↑ Cong X, Bongarzone S, Giachin G, Rossetti G, Carloni P, Legname G. Dominant-negative effects in prion diseases: insights from molecular dynamics simulations on mouse prion protein chimeras. J Biomol Struct Dyn. 2012 Aug 30. PMID:22934595 doi:10.1080/07391102.2012.712477