Aminomethyltransferase
From Proteopedia
(Difference between revisions)
| Line 14: | Line 14: | ||
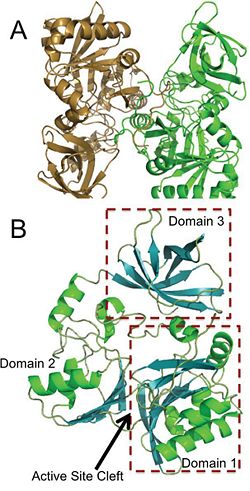
[[Image:Domains+ActiveSite.jpg|thumb|250px|left|Panel A is an image of the protein dimer DmdA. Panel B contains the three labeled domains and the active cleft site of DmdA. This image was obtained directly from Schuller et al. and is reproduced with permission of John Wiley & Sons, Inc.]] | [[Image:Domains+ActiveSite.jpg|thumb|250px|left|Panel A is an image of the protein dimer DmdA. Panel B contains the three labeled domains and the active cleft site of DmdA. This image was obtained directly from Schuller et al. and is reproduced with permission of John Wiley & Sons, Inc.]] | ||
| - | + | {{Clear}} | |
The structure of DmdA has recently been solved through the use of X-Ray diffraction <ref> Image from the RCSB PDB (www.pdb.org) of PDB ID 3TFH (Schuller, D.J., Reisch, C.R., Moran, M.A., Whitman, W.B., Lanzilotta, W.N. (2012) Structures of dimethylsulfoniopropinate-dependent demethylase from the marine organism pelagabacter ubique. Protein Sci. 21: 289-298). </ref>. The structure is a protein dimer composed of 369 amino acid residues and contains three distinct domains and four <scene name='Sandbox_Reserved_497/Ligand/1'>ligands</scene>, two of which are sodium ions and two of which are glycerol. The structure is composed of both <scene name='Sandbox_Reserved_497/Helix/1'>alpha-helices</scene> and <scene name='Sandbox_Reserved_497/Sheets/1'>beta-sheets</scene> and has <scene name='Sandbox_Reserved_497/Hydrophobic/1'>hydrophobic</scene> regions dispersed throughout the protein, though the active site cleft is highly accessible by water. The active site cleft is located between domain 1 and domain 2. Each domain contains unique identifying structural components. <scene name='Sandbox_Reserved_497/Domain1/1'>Domain 1</scene> is characterized by a Greek Key surrounded by three alpha-helices while <scene name='Sandbox_Reserved_497/Domain2/1'>domain 2</scene> contains a five-stranded antiparallel beta-sheet with alpha-helices on either side. Alternatively, <scene name='Sandbox_Reserved_497/Domain3/1'>domain 3</scene> has a distorted jellyroll formation. While DmdA belongs to the glycine cleavage T-protein (GcvT) family there is only approximately <scene name='Sandbox_Reserved_497/Conserved/1'>25% sequence identity</scene>. These few conserved amino acids likely interact with THF, which is a cofactor required by DmdA as well as many other enzymes in the GcvT family. While the exact binding mechanism of THF to the active site cleft of DmdA is still unknown, it appears as if the mechanism is unlike the general mechanism used by enzymes in the GcvT family and is unique to DmdA. In particular, amino acid residues <scene name='Sandbox_Reserved_497/Thf/1'>95, 177, 178, 204, and 206</scene> may be essential for THF binding as they assist in ring stacking as well as have the potential for hydrogen bonding. Similarly, research is still being conducted in order to determine the amino acids essential for the binding of the substrate, DMSP, to DmdA. So far it appears as if amino acid residues <scene name='Sandbox_Reserved_497/Dmsp/1'>11, 32, 197, and 246</scene> are important due to their potential for hydrogen bonding. | The structure of DmdA has recently been solved through the use of X-Ray diffraction <ref> Image from the RCSB PDB (www.pdb.org) of PDB ID 3TFH (Schuller, D.J., Reisch, C.R., Moran, M.A., Whitman, W.B., Lanzilotta, W.N. (2012) Structures of dimethylsulfoniopropinate-dependent demethylase from the marine organism pelagabacter ubique. Protein Sci. 21: 289-298). </ref>. The structure is a protein dimer composed of 369 amino acid residues and contains three distinct domains and four <scene name='Sandbox_Reserved_497/Ligand/1'>ligands</scene>, two of which are sodium ions and two of which are glycerol. The structure is composed of both <scene name='Sandbox_Reserved_497/Helix/1'>alpha-helices</scene> and <scene name='Sandbox_Reserved_497/Sheets/1'>beta-sheets</scene> and has <scene name='Sandbox_Reserved_497/Hydrophobic/1'>hydrophobic</scene> regions dispersed throughout the protein, though the active site cleft is highly accessible by water. The active site cleft is located between domain 1 and domain 2. Each domain contains unique identifying structural components. <scene name='Sandbox_Reserved_497/Domain1/1'>Domain 1</scene> is characterized by a Greek Key surrounded by three alpha-helices while <scene name='Sandbox_Reserved_497/Domain2/1'>domain 2</scene> contains a five-stranded antiparallel beta-sheet with alpha-helices on either side. Alternatively, <scene name='Sandbox_Reserved_497/Domain3/1'>domain 3</scene> has a distorted jellyroll formation. While DmdA belongs to the glycine cleavage T-protein (GcvT) family there is only approximately <scene name='Sandbox_Reserved_497/Conserved/1'>25% sequence identity</scene>. These few conserved amino acids likely interact with THF, which is a cofactor required by DmdA as well as many other enzymes in the GcvT family. While the exact binding mechanism of THF to the active site cleft of DmdA is still unknown, it appears as if the mechanism is unlike the general mechanism used by enzymes in the GcvT family and is unique to DmdA. In particular, amino acid residues <scene name='Sandbox_Reserved_497/Thf/1'>95, 177, 178, 204, and 206</scene> may be essential for THF binding as they assist in ring stacking as well as have the potential for hydrogen bonding. Similarly, research is still being conducted in order to determine the amino acids essential for the binding of the substrate, DMSP, to DmdA. So far it appears as if amino acid residues <scene name='Sandbox_Reserved_497/Dmsp/1'>11, 32, 197, and 246</scene> are important due to their potential for hydrogen bonding. | ||
| Line 23: | Line 23: | ||
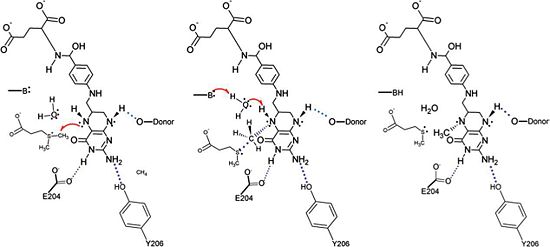
[[Image:DmdA_Mechanism.jpg|thumb|550px|right|The proposed mechanism for the methyl transfer reaction catalyzed by DmdA. This image was obtained directly from Schuller et al. and is reproduced with permission of John Wiley & Sons, Inc.]] | [[Image:DmdA_Mechanism.jpg|thumb|550px|right|The proposed mechanism for the methyl transfer reaction catalyzed by DmdA. This image was obtained directly from Schuller et al. and is reproduced with permission of John Wiley & Sons, Inc.]] | ||
| - | + | {{Clear}} | |
==Possible Applications== | ==Possible Applications== | ||
Revision as of 10:58, 2 February 2016
| |||||||||||
3D structure of aminomethyltransferase
Updated on 02-February-2016
1woo, 1wop, 1wor, 1wos – AMT – Thermotoga maritima
1vlo – EcAMT (mutant) – Escherichia coli
1v5v – AMT – Pyrococcus horikoshii
1yx2 – AMT – Bacillus subtilis
1wsr, 1wsv – AMT – human
3gir – AMT – Bartonella henselae
3tfh – CpAMT-like – Candidatus pelagibacter
3tfi – CpAMT-like + DMSP
3tfj – CpAMT-like + tetrahydrofolate
3a8i, 3a8j – EcAMT + glycine cleavage system H protein
3a8k – EcAMT (mutant) + glycine cleavage system H protein
References
- ↑ Reisch, C.R., Moran, M.A., Whitman, W.B. (2008). Dimethylsulfoniopropionate-Dependent Demethylase (DmdA) from Pelagibacter ubique and Silicibacter pomeroyi. J. Bacteriol. 190: 8018-8024.
- ↑ Malin, G. (2006). New Pieces for the Marine Sulfur Cycle Jigsaw. Science. 314: 607-608.
- ↑ Image from the RCSB PDB (www.pdb.org) of PDB ID 3TFH (Schuller, D.J., Reisch, C.R., Moran, M.A., Whitman, W.B., Lanzilotta, W.N. (2012) Structures of dimethylsulfoniopropinate-dependent demethylase from the marine organism pelagabacter ubique. Protein Sci. 21: 289-298).
- ↑ Schuller, D.J., Reisch, C.R., Moran, M.A., Whitman, W.B., Lanzilotta, W.N. (2012) Structures of dimethylsulfoniopropinate-dependent demethylase from the marine organism pelagabacter ubique. Protein Sci. 21: 289-298.
- ↑ Howard, E.C., Sun, S., Reisch, C.R., del Valle, D.A>, Burgmann, H., Kiene, R.P., and Moran, M.A. (2011). Changes in dimethylsuloniopropionate demethylase gene assemblages in response to an induced phytoplankton bloom. Appl Environ Microbiol. 77(2):524-531.
- ↑ Howard, E.C., Sun, S., Biers, E.J., and Moran, M.A. (2008). Abundant and diverse bacteria involved in DMSP degradation in marine surface waters. Environ Microbiol. 10(9);2397-2410.
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Joel L. Sussman, Kara Tinker, Jaime Prilusky