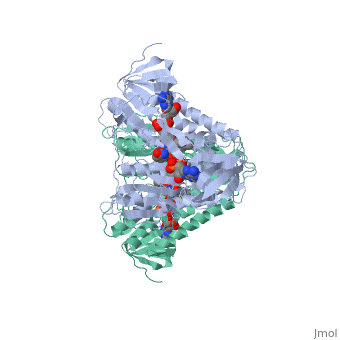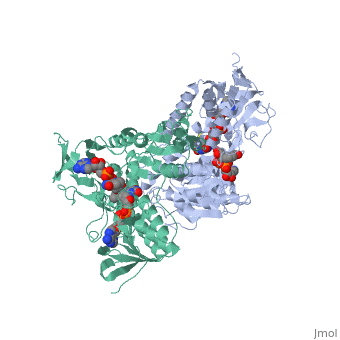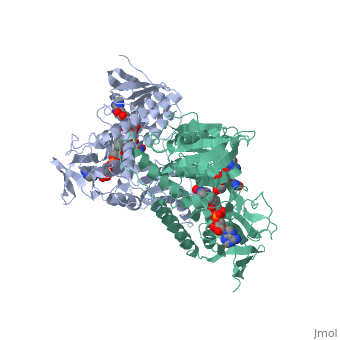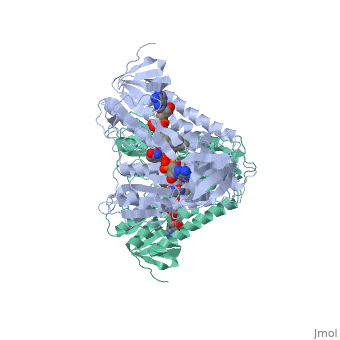
Dihydrolipoamide dehydrogenase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | < | + | <StructureSection load='1lvl' size='400' side='right' scene='' caption='FAD-containing dihydrolipoamide dehydrogenase complexed with NAD [[1lvl]]'> |
| + | |||
==General== | ==General== | ||
'''Dihyrolipoamide dehydrogenase''' (E3) is a part of the multienzyme complex of pyruvate dehydrogenase. This multienzyme complex catalyzes the formation of Acetyl-CoA from pyruvate via oxidative decarboxylation. E3 is a flavoprotein and contains FAD. | '''Dihyrolipoamide dehydrogenase''' (E3) is a part of the multienzyme complex of pyruvate dehydrogenase. This multienzyme complex catalyzes the formation of Acetyl-CoA from pyruvate via oxidative decarboxylation. E3 is a flavoprotein and contains FAD. | ||
| Line 12: | Line 13: | ||
The regulation of Dihidrolipoamide dehydrogenase (E3) kinetically comes through regulation of the entire Pyruvate Dehydrogenase complex. As would be expected, one of the main regulators is the presence of its product, acetyl-CoA as well as NADH. This is through the E1 reaction of the complex, but necessarily effects the E3 reaction. However, the E1 portion of the complex is also regulated by phosphatase and kinase in phosphorylation and dephosphorylation reactions.‘<ref>Voet, Donald et al. 2008. Fundamentals of Biochemistry. 3rd ed. p.585</ref>’ | The regulation of Dihidrolipoamide dehydrogenase (E3) kinetically comes through regulation of the entire Pyruvate Dehydrogenase complex. As would be expected, one of the main regulators is the presence of its product, acetyl-CoA as well as NADH. This is through the E1 reaction of the complex, but necessarily effects the E3 reaction. However, the E1 portion of the complex is also regulated by phosphatase and kinase in phosphorylation and dephosphorylation reactions.‘<ref>Voet, Donald et al. 2008. Fundamentals of Biochemistry. 3rd ed. p.585</ref>’ | ||
| - | + | </StructureSection> | |
==3D structures of dihydrolipoamide dehydrogenase== | ==3D structures of dihydrolipoamide dehydrogenase== | ||
Revision as of 12:31, 2 February 2016
| |||||||||||
3D structures of dihydrolipoamide dehydrogenase
Updated on 02-February-2016
References
- ↑ Brautigam CA, Wynn RM, Chuang JL, Machius M, Tomchick DR, Chuang DT. Structural insight into interactions between dihydrolipoamide dehydrogenase (E3) and E3 binding protein of human pyruvate dehydrogenase complex. Structure. 2006 Mar;14(3):611-21. Epub 2006 Jan 26. PMID:16442803 doi:10.1016/j.str.2006.01.001
- ↑ Voet, Donald et al. 2008. Fundamentals of Biochemistry. 3rd ed. pp.570-575
- ↑ Voet, Donald et al. 2008. Fundamentals of Biochemistry. 3rd ed. p.585
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Nicholas Rockefeller, Alexander Berchansky, David Canner, Shane Michael Evans, Jaime Prilusky