1d5f
From Proteopedia
| Line 7: | Line 7: | ||
|ACTIVITY= | |ACTIVITY= | ||
|GENE= | |GENE= | ||
| + | |DOMAIN= | ||
| + | |RELATEDENTRY=[[1ubq|1UBQ]] | ||
| + | |RESOURCES=<span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1d5f FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1d5f OCA], [http://www.ebi.ac.uk/pdbsum/1d5f PDBsum], [http://www.rcsb.org/pdb/explore.do?structureId=1d5f RCSB]</span> | ||
}} | }} | ||
| Line 14: | Line 17: | ||
==Overview== | ==Overview== | ||
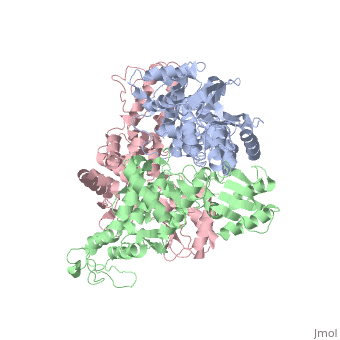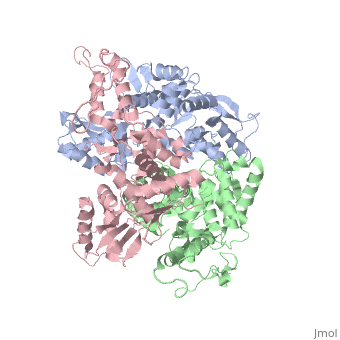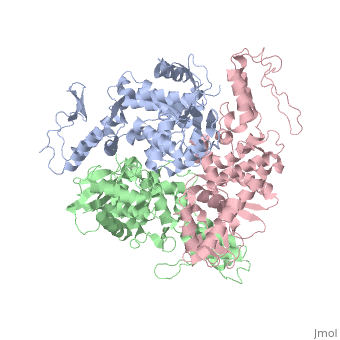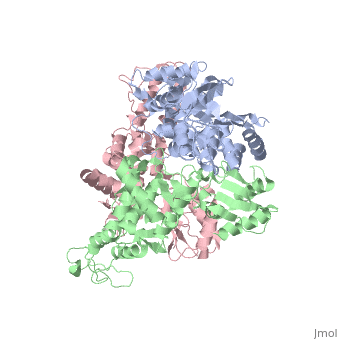
The E6AP ubiquitin-protein ligase (E3) mediates the human papillomavirus-induced degradation of the p53 tumor suppressor in cervical cancer and is mutated in Angelman syndrome, a neurological disorder. The crystal structure of the catalytic hect domain of E6AP reveals a bilobal structure with a broad catalytic cleft at the junction of the two lobes. The cleft consists of conserved residues whose mutation interferes with ubiquitin-thioester bond formation and is the site of Angelman syndrome mutations. The crystal structure of the E6AP hect domain bound to the UbcH7 ubiquitin-conjugating enzyme (E2) reveals the determinants of E2-E3 specificity and provides insights into the transfer of ubiquitin from the E2 to the E3. | The E6AP ubiquitin-protein ligase (E3) mediates the human papillomavirus-induced degradation of the p53 tumor suppressor in cervical cancer and is mutated in Angelman syndrome, a neurological disorder. The crystal structure of the catalytic hect domain of E6AP reveals a bilobal structure with a broad catalytic cleft at the junction of the two lobes. The cleft consists of conserved residues whose mutation interferes with ubiquitin-thioester bond formation and is the site of Angelman syndrome mutations. The crystal structure of the E6AP hect domain bound to the UbcH7 ubiquitin-conjugating enzyme (E2) reveals the determinants of E2-E3 specificity and provides insights into the transfer of ubiquitin from the E2 to the E3. | ||
| - | |||
| - | ==Disease== | ||
| - | Known disease associated with this structure: Angelman syndrome OMIM:[[http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=601623 601623]] | ||
==About this Structure== | ==About this Structure== | ||
| Line 36: | Line 36: | ||
[[Category: elongated shape]] | [[Category: elongated shape]] | ||
| - | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Sun Mar 30 19:35:03 2008'' |
Revision as of 16:35, 30 March 2008
| |||||||
| , resolution 2.8Å | |||||||
|---|---|---|---|---|---|---|---|
| Related: | 1UBQ
| ||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||
STRUCTURE OF AN E6AP-UBCH7 COMPLEX: INSIGHTS INTO THE UBIQUITINATION PATHWAY
Overview
The E6AP ubiquitin-protein ligase (E3) mediates the human papillomavirus-induced degradation of the p53 tumor suppressor in cervical cancer and is mutated in Angelman syndrome, a neurological disorder. The crystal structure of the catalytic hect domain of E6AP reveals a bilobal structure with a broad catalytic cleft at the junction of the two lobes. The cleft consists of conserved residues whose mutation interferes with ubiquitin-thioester bond formation and is the site of Angelman syndrome mutations. The crystal structure of the E6AP hect domain bound to the UbcH7 ubiquitin-conjugating enzyme (E2) reveals the determinants of E2-E3 specificity and provides insights into the transfer of ubiquitin from the E2 to the E3.
About this Structure
1D5F is a Single protein structure of sequence from Homo sapiens. Full crystallographic information is available from OCA.
Reference
Structure of an E6AP-UbcH7 complex: insights into ubiquitination by the E2-E3 enzyme cascade., Huang L, Kinnucan E, Wang G, Beaudenon S, Howley PM, Huibregtse JM, Pavletich NP, Science. 1999 Nov 12;286(5443):1321-6. PMID:10558980
Page seeded by OCA on Sun Mar 30 19:35:03 2008