Hormone sensitive lipase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
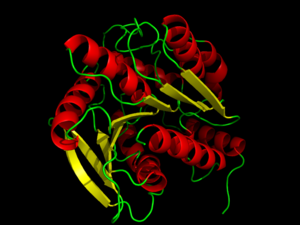
| - | <StructureSection load='3DNM' size=' | + | <StructureSection load='3DNM' size='350' side='right' caption='Hormone-Sensitive Lipase (PDB: [[3dnm]])' scene='58/580297/3dnm_cartoon/2' > |
==Introduction== | ==Introduction== | ||
| Line 7: | Line 7: | ||
Hormone-sensitive [http://proteopedia.org/wiki/index.php/Lipase lipases] (HSL) represent a class of [http://en.wikipedia.org/wiki/Esterase esterases] within the [http://www.proteopedia.org/wiki/index.php/Hydrolase α/β hydrolase] family. HSL catalyzes the cleavage of ester bonds in fatty acid molecules when stimulated by a hormone.<ref name="Holm">PMID:14641008</ref> The activation and mobilization of hormone-sensitive lipase can be triggered by various [http://en.wikipedia.org/wiki/Catecholamine catecholamines] and inhibited by [http://www.proteopedia.org/wiki/index.php/Insulin insulin].<ref name= "Ray">PMID:12765952</ref> Catecholamines, such as [http://en.wikipedia.org/wiki/Epinephrine epinephrine], are rapidly spread throughout the body during times of energy mobilization, like in the [http://en.wikipedia.org/wiki/Fight-or-flight_response fight or flight response]. Conversely, insulin triggers glucose uptake, requiring the storage of energy, opposing HSL's function. HSL is clinically relevant, because the mobilization of or inability to mobilize fats in cells is directly related to fat accumulation seen in [http://en.wikipedia.org/wiki/Atherosclerosis artherosclerosis]and [http://en.wikipedia.org/wiki/Obesity obesity].<ref name= "Yeaman">PMID:14725507</ref> Such diseases are characterized by an accumulation of fats and researchers are investigating whether HSL's activity plays a role or not.<ref name= "Ray">PMID:12765952</ref> Investigation of HSL's structure and function could provide a better clinical understanding of these diseases.<ref name= "Yeaman">PMID:14725507</ref> | Hormone-sensitive [http://proteopedia.org/wiki/index.php/Lipase lipases] (HSL) represent a class of [http://en.wikipedia.org/wiki/Esterase esterases] within the [http://www.proteopedia.org/wiki/index.php/Hydrolase α/β hydrolase] family. HSL catalyzes the cleavage of ester bonds in fatty acid molecules when stimulated by a hormone.<ref name="Holm">PMID:14641008</ref> The activation and mobilization of hormone-sensitive lipase can be triggered by various [http://en.wikipedia.org/wiki/Catecholamine catecholamines] and inhibited by [http://www.proteopedia.org/wiki/index.php/Insulin insulin].<ref name= "Ray">PMID:12765952</ref> Catecholamines, such as [http://en.wikipedia.org/wiki/Epinephrine epinephrine], are rapidly spread throughout the body during times of energy mobilization, like in the [http://en.wikipedia.org/wiki/Fight-or-flight_response fight or flight response]. Conversely, insulin triggers glucose uptake, requiring the storage of energy, opposing HSL's function. HSL is clinically relevant, because the mobilization of or inability to mobilize fats in cells is directly related to fat accumulation seen in [http://en.wikipedia.org/wiki/Atherosclerosis artherosclerosis]and [http://en.wikipedia.org/wiki/Obesity obesity].<ref name= "Yeaman">PMID:14725507</ref> Such diseases are characterized by an accumulation of fats and researchers are investigating whether HSL's activity plays a role or not.<ref name= "Ray">PMID:12765952</ref> Investigation of HSL's structure and function could provide a better clinical understanding of these diseases.<ref name= "Yeaman">PMID:14725507</ref> | ||
| - | + | ==Activation of HSL== | |
Briefly, HSL is stimulated by the binding of catecholamines to β-adrenergic receptors. β-adrenergic receptors coupled with [http://www.proteopedia.org/wiki/index.php/Adenylate_Cyclase adenylate cyclase] (AC) then stimulate G-proteins to increase the levels of cystolic [http://en.wikipedia.org/wiki/Cyclic_adenosine_monophosphate cAMP]. Elevated levels of cAMP leads to an activation [http://www.proteopedia.org/wiki/index.php/Protein_kinase_A protein kinase A] (PKA) leading to phosphorylation of serine residues on HSL activating and translocating HSL to lipid droplets for lipolysis. Conversely, insulin signaling decreases cystolic cAMP levels, resulting in a decreased HSL mobilization.<ref name="Holm">PMID:14641008</ref> | Briefly, HSL is stimulated by the binding of catecholamines to β-adrenergic receptors. β-adrenergic receptors coupled with [http://www.proteopedia.org/wiki/index.php/Adenylate_Cyclase adenylate cyclase] (AC) then stimulate G-proteins to increase the levels of cystolic [http://en.wikipedia.org/wiki/Cyclic_adenosine_monophosphate cAMP]. Elevated levels of cAMP leads to an activation [http://www.proteopedia.org/wiki/index.php/Protein_kinase_A protein kinase A] (PKA) leading to phosphorylation of serine residues on HSL activating and translocating HSL to lipid droplets for lipolysis. Conversely, insulin signaling decreases cystolic cAMP levels, resulting in a decreased HSL mobilization.<ref name="Holm">PMID:14641008</ref> | ||
Revision as of 10:50, 8 February 2016
| |||||||||||
Proteopedia Pages
References
- ↑ 1.0 1.1 Holm C. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem Soc Trans. 2003 Dec;31(Pt 6):1120-4. PMID:14641008 doi:http://dx.doi.org/10.1042/
- ↑ 2.0 2.1 Ray H, Beylot M, Arner P, Larrouy D, Langin D, Holm C, Large V. The presence of a catalytically inactive form of hormone-sensitive lipase is associated with decreased lipolysis in abdominal subcutaneous adipose tissue of obese subjects. Diabetes. 2003 Jun;52(6):1417-22. PMID:12765952
- ↑ 3.0 3.1 3.2 3.3 3.4 Yeaman SJ. Hormone-sensitive lipase--new roles for an old enzyme. Biochem J. 2004 Apr 1;379(Pt 1):11-22. PMID:14725507 doi:http://dx.doi.org/10.1042/BJ20031811
- ↑ 4.0 4.1 4.2 4.3 4.4 Nam KH, Kim MY, Kim SJ, Priyadarshi A, Kwon ST, Koo BS, Yoon SH, Hwang KY. Structural and functional analysis of a novel hormone-sensitive lipase from a metagenome library. Proteins. 2009 Mar;74(4):1036-40. PMID:19089974 doi:http://dx.doi.org/10.1002/prot.22313
- ↑ 5.0 5.1 Kanwar SS, Kaushal RK, Jawed A, Gupta R, Chimni SS. Methods for inhibition of residual lipase activity in colorimetric assay: a comparative study. Indian J Biochem Biophys. 2005 Aug;42(4):233-7. PMID:23923547
- ↑ 6.0 6.1 Nam KH, Kim SJ, Priyadarshi A, Kim HS, Hwang KY. The crystal structure of an HSL-homolog EstE5 complex with PMSF reveals a unique configuration that inhibits the nucleophile Ser144 in catalytic triads. Biochem Biophys Res Commun. 2009 Nov 13;389(2):247-50. Epub 2009 Aug 26. PMID:19715665 doi:10.1016/j.bbrc.2009.08.123
- ↑ Kraemer FB, Shen WJ. Hormone-sensitive lipase: control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. J Lipid Res. 2002 Oct;43(10):1585-94. PMID:12364542
Student Contributors
Nathan Holt
Derek O'Connor
Proteopedia Page Contributors and Editors (what is this?)
Karsten Theis, R. Jeremy Johnson, Angel Herraez, Michal Harel