Hemoglobin
From Proteopedia
| Line 1: | Line 1: | ||
==Section1 == | ==Section1 == | ||
| - | <applet load="1gzx" size=" | + | <applet load="1gzx" size="200" color="white" frame="true" align="right" caption="Hemoglobin" /> |
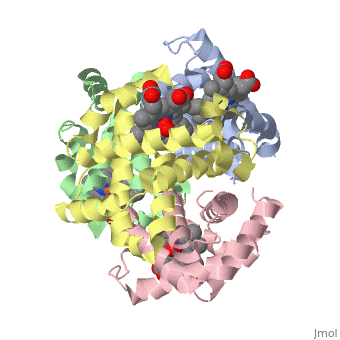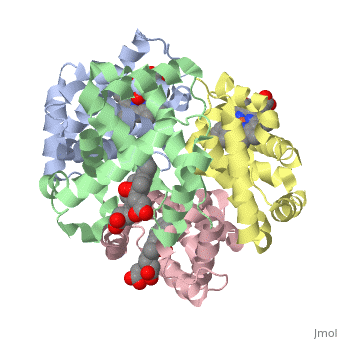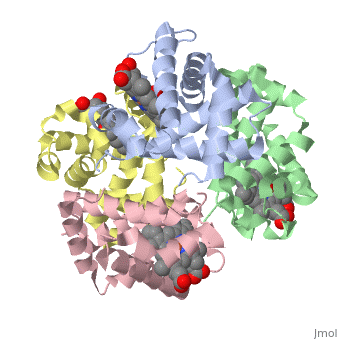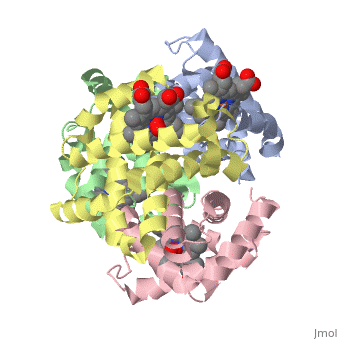
'''Hemoglobin''' is an oxygen-transport protein. Hemoglobin is an [[allosteric protein]]. It is a tetramer composed of two types of subunits designated α and β, whose stoichiometry is <scene name='Hemoglobin/Alpha2beta2/2'>α2β2</scene>. The | '''Hemoglobin''' is an oxygen-transport protein. Hemoglobin is an [[allosteric protein]]. It is a tetramer composed of two types of subunits designated α and β, whose stoichiometry is <scene name='Hemoglobin/Alpha2beta2/2'>α2β2</scene>. The | ||
Revision as of 08:14, 16 October 2007
Section1
|
Hemoglobin is an oxygen-transport protein. Hemoglobin is an allosteric protein. It is a tetramer composed of two types of subunits designated α and β, whose stoichiometry is . The of hemoglobin sit roughly at the corners of a tetrahedron, facing each other across a at the center of the molecule. Each of the subunits prosthetic group. The give hemoglobin its red color.
Each individual molecule contains one atom. In the lungs, where oxygen is abundant, an binds to the ferrous iron atom of the heme molecule and is later released in tissues needing oxygen. The heme group binds oxygen while still attached to the . The spacefill view of the hemoglobin polypeptide subunit with an oxygenated heme group shows how the within the polypeptide.
is facilitated by a histidine nitrogen that binds to the iron. A second histidine is near the bound oxygen. The "arms" (propanoate groups) of heme face are hydrophilic and face the surface of the protein while the hydrophobic portions of the heme are buried among the hydrophobic amino acids of the protein. We can also view the anchored heme with the hemoglobin polypeptide subunit shown in a representation.
Section 2
|
Hemoglobin is an oxygen-transport protein. Hemoglobin is an allosteric protein. It is a tetramer composed of two types of subunits designated α and β, whose stoichiometry is . The of hemoglobin sit roughly at the corners of a tetrahedron, facing each other across a at the center of the molecule. Each of the subunits prosthetic group. The give hemoglobin its red color.
Each individual molecule contains one atom. In the lungs, where oxygen is abundant, an binds to the ferrous iron atom of the heme molecule and is later released in tissues needing oxygen. The heme group binds oxygen while still attached to the . The spacefill view of the hemoglobin polypeptide subunit with an oxygenated heme group shows how the within the polypeptide.
is facilitated by a histidine nitrogen that binds to the iron. A second histidine is near the bound oxygen. The "arms" (propanoate groups) of heme face are hydrophilic and face the surface of the protein while the hydrophobic portions of the heme are buried among the hydrophobic amino acids of the protein. We can also view the anchored heme with the hemoglobin polypeptide subunit shown in a representation.
Proteopedia Page Contributors and Editors (what is this?)
Eran Hodis, Michal Harel, Joel L. Sussman, Alexander Berchansky, Jaime Prilusky, Karsten Theis, Eric Martz, Karl Oberholser, Tihitina Y Aytenfisu, Mark Hoelzer, Marc Gillespie, Ann Taylor, Marius Mihasan, Manisha Chawda, Hannah Campbell