Succinyl-CoA synthetase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
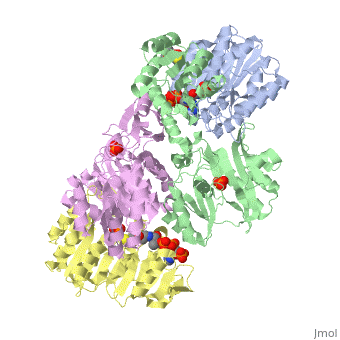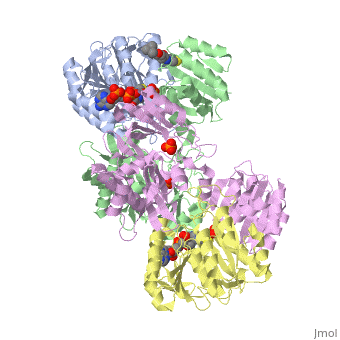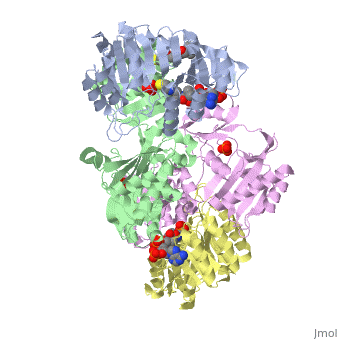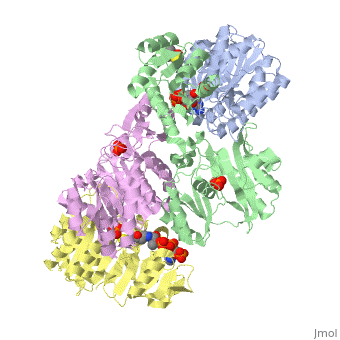
| - | <StructureSection load='1cqj' size=' | + | <StructureSection load='1cqj' size='350' side='right' scene='' caption='E. coli succinyl-CoA synthetase α subunit (grey, yellow) and β subunit (green, magenta) complex with CoA and phosphate (PDB code [[1cqj]])'> |
| - | '''Succinyl-Coa synthetase''' catalyzes the reversible reaction of succinyl-CoA + NDP + Pi <-> succinate + CoA + NTP (where N is either adenosine or guanosine. It can be found in Escherichia coli and is the fifth step in the [[Citric Acid Cycle|citric acid cycle]]. Due to its involvement in the citric acid cycle it is very important in all living cells that use oxygen as a part of cellular respiration. This entire process (Citric acid cycle) is necessary in producing one GTP or ATP, three NADHs, and two carbon dioxides. | + | '''Succinyl-Coa synthetase''' catalyzes the reversible reaction of succinyl-CoA + NDP + Pi <-> succinate + CoA + NTP (where N is either adenosine or guanosine. It can be found in Escherichia coli and is the fifth step in the [[Citric Acid Cycle|citric acid cycle]]. Due to its involvement in the citric acid cycle it is very important in all living cells that use oxygen as a part of cellular respiration. This entire process (Citric acid cycle) is necessary in producing one GTP or ATP, three NADHs, and two carbon dioxides. See also:<br /> |
| + | *[[Krebs cycle carbons]] | ||
| + | *[[Krebs cycle importance]] | ||
| + | *[[Krebs cycle overview]] | ||
| + | *[[Krebs cycle step 5]] | ||
==Structure== | ==Structure== | ||
Revision as of 09:58, 11 February 2016
| |||||||||||
3D structures of succinyl-CoA synthetase
Updated on 11-February-2016
Additional Resources
References
- ↑ Joyce MA, Fraser ME, Brownie ER, James MN, Bridger WA, Wolodko WT. Probing the nucleotide-binding site of Escherichia coli succinyl-CoA synthetase. Biochemistry. 1999 Jun 1;38(22):7273-83. PMID:10353839 doi:10.1021/bi990527s
- ↑ Fraser ME, Joyce MA, Ryan DG, Wolodko WT. Two glutamate residues, Glu 208 alpha and Glu 197 beta, are crucial for phosphorylation and dephosphorylation of the active-site histidine residue in succinyl-CoA synthetase. Biochemistry. 2002 Jan 15;41(2):537-46. PMID:11781092
- ↑ Bild GS, Janson CA, Boyer PD. Subunit interaction during catalysis. ATP modulation of catalytic steps in the succinyl-CoA synthetase reaction. J Biol Chem. 1980 Sep 10;255(17):8109-15. PMID:6997289
- ↑ Mikeladze DG, Matveeva LN, Severin SE. [Reaction mechanism of succinyl CoA synthetase from pigeon thoracic muscle] Biokhimiia. 1978 Aug;43(8):1458-67. PMID:570066
- ↑ Joyce MA, Fraser ME, James MN, Bridger WA, Wolodko WT. ADP-binding site of Escherichia coli succinyl-CoA synthetase revealed by x-ray crystallography. Biochemistry. 2000 Jan 11;39(1):17-25. PMID:10625475
- ↑ Frank J. Moffet and W. A. Bridger. The Kinetics of Succinyl Coenzyme A Synthetase from Escherichia coli : A REACTION WITH A COVALENT ENZYME-SUBSTRATE INTERMEDIATE NOT EXHIBITING "PING-PONG" KINETICS J. Biol. Chem. 1970 245: 2758-2762.[1]
- ↑ Um, H.-D., and C. Klein. 1993. Evidence for allosteric regulation of succinyl-CoA synthetase. Biochem. J. 295:821–826.[2]
Content Donators
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Lucas Hamlow, J.D. McClintic, Wayne Decatur, David Canner, Alexander Berchansky