We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
GTPase HRas
From Proteopedia
(Difference between revisions)
| Line 20: | Line 20: | ||
[[Chani elisha]]. | [[Chani elisha]]. | ||
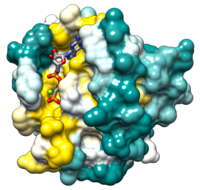
| - | The two most common oncogenic mutations in ''H-RAS'' affect residues <scene name='User:Joseph_Lipsick/RAS/Ras-gtp_gly12/1'>Gly12 </scene> and <scene name='User:Joseph_Lipsick/RAS/Ras-gtp_gln61/1'>Gln61</scene>, both of which are adjacent to the bound GTP molecule. These oncogenic mutations greatly inhibit the intrinsic GTPase activity, thereby causing the RAS switch to spend more time in the "ON" position. The RAS proteins are present at the plasma membrane and transmit signals from transmembrance receptor tyrosine kinases (e.g. EGF and PDGF receptors) to downstream intracellular effectors that include the MAPK protein kinase cascade and the [[PI3K|PI3K lipid kinase]]. Binding of EGF or PDGF to their receptors causes a relocalization of a '''G'''uanine Nucleotide '''E'''xchange '''F'''actor (GEF) protein to the plasma membrane. The structure of <scene name='User:Joseph_Lipsick/RAS/Ras-sos/5'>SOS</scene>, a prototypic GEF, together with RAS implies that the GEF prys open the nucleotide binding site with a loss of bound GDP. The ten-fold higher ratio of GTP to GDP within the cell results in the replacement of RAS-GDP ("OFF" state) with RAS-GTP ("ON" state). Consistent with this model, gain-of-function mutations of ''GEF'' genes can be oncogenic (e.g. ''VAV'' oncogene) even in the absence of mutations of ''RAS''. Conversely, a GTPase Activating Protein (<scene name='User:Joseph_Lipsick/RAS/Ras-gap/5'>GAP</scene>) can bind to RAS-GTP and increase the rate of the intrinsic RAS GTPase. Consistent with this model, loss-of-function mutations of GAP genes (e.g. NF1 tumor suppressor) can be oncogenic even in the absence of mutations of ''RAS'' itself. More details in [[Allosteric modulation of H-Ras GTPase]]. | + | The two most common oncogenic mutations in ''H-RAS'' affect residues <scene name='User:Joseph_Lipsick/RAS/Ras-gtp_gly12/1'>Gly12 </scene> and <scene name='User:Joseph_Lipsick/RAS/Ras-gtp_gln61/1'>Gln61</scene>, both of which are adjacent to the bound GTP molecule. These oncogenic mutations greatly inhibit the intrinsic GTPase activity, thereby causing the RAS switch to spend more time in the "ON" position. The RAS proteins are present at the plasma membrane and transmit signals from transmembrance receptor tyrosine kinases (e.g. EGF and PDGF receptors) to downstream intracellular effectors that include the MAPK protein kinase cascade and the [[PI3K|PI3K lipid kinase]]. Binding of EGF or PDGF to their receptors causes a relocalization of a '''G'''uanine Nucleotide '''E'''xchange '''F'''actor (GEF) protein to the plasma membrane. The structure of <scene name='User:Joseph_Lipsick/RAS/Ras-sos/5'>SOS</scene>, a prototypic GEF, together with RAS implies that the GEF prys open the nucleotide binding site with a loss of bound GDP. The ten-fold higher ratio of GTP to GDP within the cell results in the replacement of RAS-GDP ("OFF" state) with RAS-GTP ("ON" state). Consistent with this model, gain-of-function mutations of ''GEF'' genes can be oncogenic (e.g. ''VAV'' oncogene) even in the absence of mutations of ''RAS''. Conversely, a GTPase Activating Protein (<scene name='User:Joseph_Lipsick/RAS/Ras-gap/5'>GAP</scene>) can bind to RAS-GTP and increase the rate of the intrinsic RAS GTPase. Consistent with this model, loss-of-function mutations of GAP genes (e.g. NF1 tumor suppressor) can be oncogenic even in the absence of mutations of ''RAS'' itself. More details in<br /> |
| + | [[Allosteric modulation of H-Ras GTPase]]<br /> | ||
| + | [[Proteins involved in cancer]]. | ||
</StructureSection> | </StructureSection> | ||
Revision as of 09:37, 18 February 2016
| |||||||||||
3D structures of GTPase Hras
Updated on 18-February-2016
References
- Milburn, et al. Science. 1990. 247: 939-45.
- Wittinghofer and Pai. Trends Biochem Sci. 1991. 16: 382-7.
- Malumbres and Barbacid. Nature Rev Cancer. 2003. 3: 459-65.
- Bos, Rehmann, and Wittinghofer. Cell. 2007. 129: 865-77.
- Karnoub and Weinberg. Nature Rev Mol Cell Biol. 2008. 9: 517-531.
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, David Canner, Alexander Berchansky, Joel L. Sussman, Joseph Lipsick, Jaime Prilusky