We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
RuBisCO
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| + | == Function == | ||
| + | |||
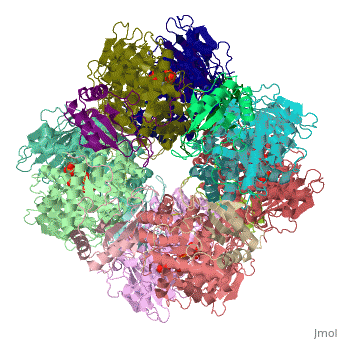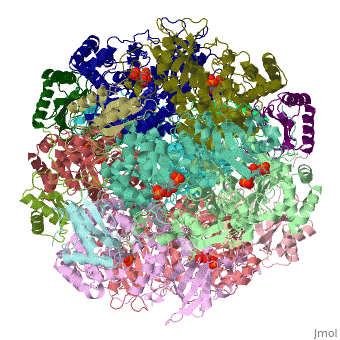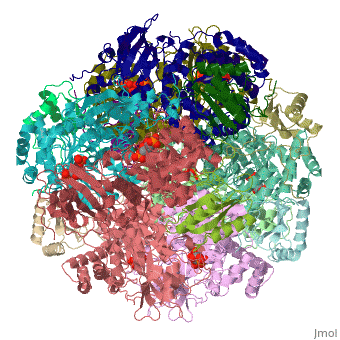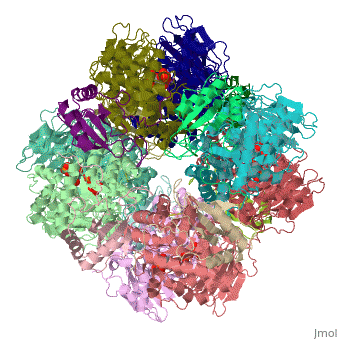
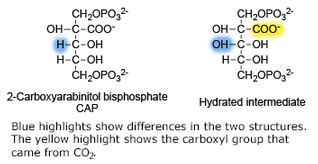
'''Ribulose-1,5-bisphosphate carboxylase oxygenase – RuBisCO''' (RBCO) catalyzes the first step in photosynthetic carbon fixation, and it is the most abundant protein on earth. RBCO can either carboxylate or oxygenate ribulose-1,5-bisphosphate (RUBP) with CO<sub>2</sub> or O<sub>2</sub>, respectively. RBCO from flowering plants consists of eight large subunits and eight small subunits. | '''Ribulose-1,5-bisphosphate carboxylase oxygenase – RuBisCO''' (RBCO) catalyzes the first step in photosynthetic carbon fixation, and it is the most abundant protein on earth. RBCO can either carboxylate or oxygenate ribulose-1,5-bisphosphate (RUBP) with CO<sub>2</sub> or O<sub>2</sub>, respectively. RBCO from flowering plants consists of eight large subunits and eight small subunits. | ||
Revision as of 08:55, 21 February 2016
Contents |
Function
Ribulose-1,5-bisphosphate carboxylase oxygenase – RuBisCO (RBCO) catalyzes the first step in photosynthetic carbon fixation, and it is the most abundant protein on earth. RBCO can either carboxylate or oxygenate ribulose-1,5-bisphosphate (RUBP) with CO2 or O2, respectively. RBCO from flowering plants consists of eight large subunits and eight small subunits.
Structural Features
| |||||||||||
3D structures of RuBisCO
Updated on 21-February-2016
See Also
References
- ↑ Andersson I. Large structures at high resolution: the 1.6 A crystal structure of spinach ribulose-1,5-bisphosphate carboxylase/oxygenase complexed with 2-carboxyarabinitol bisphosphate. J Mol Biol. 1996 May 31;259(1):160-74. PMID:8648644 doi:10.1006/jmbi.1996.0310
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alice Harmon, Joel L. Sussman, Alexander Berchansky