Vascular Endothelial Growth Factor Receptor
From Proteopedia
(Difference between revisions)
| Line 24: | Line 24: | ||
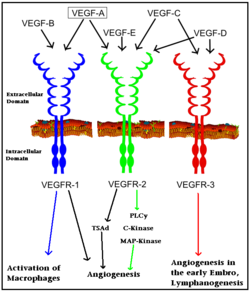
VEGFRs play a critical role in a number of signal transduction pathways essential for angiogenesis and cell migration. VEGFR is a particularly attractive target because they are expressed almost exclusively in endothelial cells and are highly upregulated in many tumor endothelium types.<ref>PMID:12360282</ref> In fact, work by Plate ''et al.'' revealed that VEGFR-2 expression is 5 fold higher in the tumor vasculature than in normal vasculature. This increased VEGFR-2 expression is due to a cancer cells high metabolic demand for oxygen and other nutrients to continue growing, thus requiring a vast vasculature. VEGFR signaling has also been implicated in diabetic reinopathy and the progression of rheumatoid arthritis and atherosclerosis.<ref>PMID:17826917 </ref> | VEGFRs play a critical role in a number of signal transduction pathways essential for angiogenesis and cell migration. VEGFR is a particularly attractive target because they are expressed almost exclusively in endothelial cells and are highly upregulated in many tumor endothelium types.<ref>PMID:12360282</ref> In fact, work by Plate ''et al.'' revealed that VEGFR-2 expression is 5 fold higher in the tumor vasculature than in normal vasculature. This increased VEGFR-2 expression is due to a cancer cells high metabolic demand for oxygen and other nutrients to continue growing, thus requiring a vast vasculature. VEGFR signaling has also been implicated in diabetic reinopathy and the progression of rheumatoid arthritis and atherosclerosis.<ref>PMID:17826917 </ref> | ||
| - | Bevacizumab ([[Avastin]]) is a recombinant [[monoclonal antibody]] marketed by the pharmaceutical company Roche. It earned over $5 billion dollars in 2009 treating a number of cancers. It’s principal mechanism of action is as an anti-VEGF antibody that favors antiangiogenesis in the tumor microenvironment while effecting the rest of the body to a lesser extent. It has been found to decrease tumor vascular permeability subsequently reducing the delivery of oxygen and nutrients to cancer cells when used in combination with chemotherapy.<ref>PMID:11533692 </ref> Other drugs target VEGFR such as [[Sorafenib]] ([[Nexavar]]), Sunitinib ([[Sutent]]) and Vandetanib, binding to various parts of the receptor, either preventing interaction with [[VEGF]] or with other downstream signaling molecules.<ref>PMID:17826917 </ref> | + | Bevacizumab ([[Avastin]]) is a recombinant [[monoclonal antibody]] marketed by the pharmaceutical company Roche. It earned over $5 billion dollars in 2009 treating a number of cancers. It’s principal mechanism of action is as an anti-VEGF antibody that favors antiangiogenesis in the tumor microenvironment while effecting the rest of the body to a lesser extent. It has been found to decrease tumor vascular permeability subsequently reducing the delivery of oxygen and nutrients to cancer cells when used in combination with chemotherapy.<ref>PMID:11533692 </ref> Other drugs target VEGFR such as [[Sorafenib]] ([[Nexavar]]), [[Sunitinib]] ([[Sutent]]) and Vandetanib, binding to various parts of the receptor, either preventing interaction with [[VEGF]] or with other downstream signaling molecules.<ref>PMID:17826917 </ref> |
<br /> | <br /> | ||
</StructureSection> | </StructureSection> | ||
Revision as of 11:43, 22 February 2016
| |||||||||||
3D Structures of VEGFR
Updated on 22-February-2016
Additional Resources
For additional information, see: Cancer
See Also
References
- ↑ Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006 May;7(5):359-71. PMID:16633338 doi:10.1038/nrm1911
- ↑ Holmes K, Roberts OL, Thomas AM, Cross MJ. Vascular endothelial growth factor receptor-2: structure, function, intracellular signalling and therapeutic inhibition. Cell Signal. 2007 Oct;19(10):2003-12. Epub 2007 Jun 12. PMID:17658244 doi:10.1016/j.cellsig.2007.05.013
- ↑ Gallina P, Nohra G, Cioloca C, Meder JF, Roux FX. [Multiple cavernoma of delayed appearance] Neurochirurgie. 1994;40(5):322-5. PMID:7596453
- ↑ Shinkai A, Ito M, Anazawa H, Yamaguchi S, Shitara K, Shibuya M. Mapping of the sites involved in ligand association and dissociation at the extracellular domain of the kinase insert domain-containing receptor for vascular endothelial growth factor. J Biol Chem. 1998 Nov 20;273(47):31283-8. PMID:9813036
- ↑ Ruch C, Skiniotis G, Steinmetz MO, Walz T, Ballmer-Hofer K. Structure of a VEGF-VEGF receptor complex determined by electron microscopy. Nat Struct Mol Biol. 2007 Mar;14(3):249-50. Epub 2007 Feb 11. PMID:17293873 doi:10.1038/nsmb1202
- ↑ Shibuya M, Yamaguchi S, Yamane A, Ikeda T, Tojo A, Matsushime H, Sato M. Nucleotide sequence and expression of a novel human receptor-type tyrosine kinase gene (flt) closely related to the fms family. Oncogene. 1990 Apr;5(4):519-24. PMID:2158038
- ↑ Ji QS, Winnier GE, Niswender KD, Horstman D, Wisdom R, Magnuson MA, Carpenter G. Essential role of the tyrosine kinase substrate phospholipase C-gamma1 in mammalian growth and development. Proc Natl Acad Sci U S A. 1997 Apr 1;94(7):2999-3003. PMID:9096335
- ↑ Welsh M, Songyang Z, Frantz JD, Trub T, Reedquist KA, Karlsson T, Miyazaki M, Cantley LC, Band H, Shoelson SE. Stimulation through the T cell receptor leads to interactions between SHB and several signaling proteins. Oncogene. 1998 Feb 19;16(7):891-901. PMID:9484780 doi:10.1038/sj.onc.1201607
- ↑ Zeng H, Sanyal S, Mukhopadhyay D. Tyrosine residues 951 and 1059 of vascular endothelial growth factor receptor-2 (KDR) are essential for vascular permeability factor/vascular endothelial growth factor-induced endothelium migration and proliferation, respectively. J Biol Chem. 2001 Aug 31;276(35):32714-9. Epub 2001 Jul 2. PMID:11435426 doi:10.1074/jbc.M103130200
- ↑ Chen M, She H, Davis EM, Spicer CM, Kim L, Ren R, Le Beau MM, Li W. Identification of Nck family genes, chromosomal localization, expression, and signaling specificity. J Biol Chem. 1998 Sep 25;273(39):25171-8. PMID:9737977
- ↑ Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002 Oct;2(10):795-803. PMID:12360282 doi:10.1038/nrc909
- ↑ Rosa DD, Ismael G, Lago LD, Awada A. Molecular-targeted therapies: lessons from years of clinical development. Cancer Treat Rev. 2008 Feb;34(1):61-80. Epub 2007 Sep 10. PMID:17826917 doi:10.1016/j.ctrv.2007.07.019
- ↑ Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001 Sep;7(9):987-9. PMID:11533692 doi:10.1038/nm0901-987
- ↑ Rosa DD, Ismael G, Lago LD, Awada A. Molecular-targeted therapies: lessons from years of clinical development. Cancer Treat Rev. 2008 Feb;34(1):61-80. Epub 2007 Sep 10. PMID:17826917 doi:10.1016/j.ctrv.2007.07.019
Proteopedia Page Contributors and Editors (what is this?)
David Canner, Michal Harel, Joel L. Sussman, Alexander Berchansky, Jaime Prilusky, Wayne Decatur