This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Glycogen synthase kinase 3
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
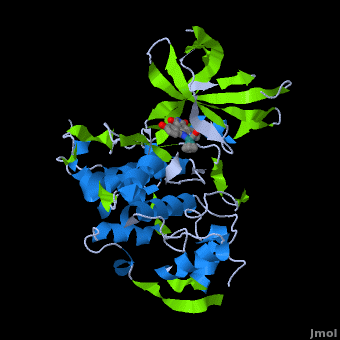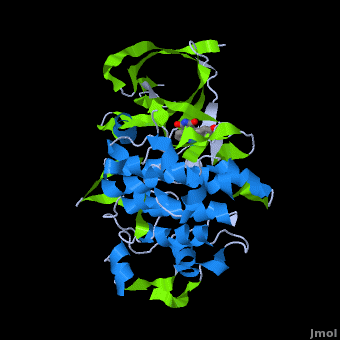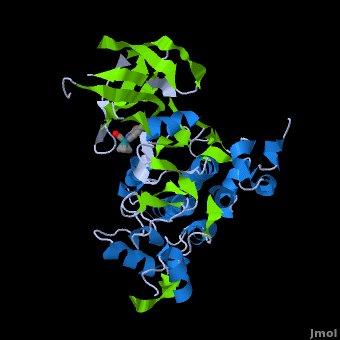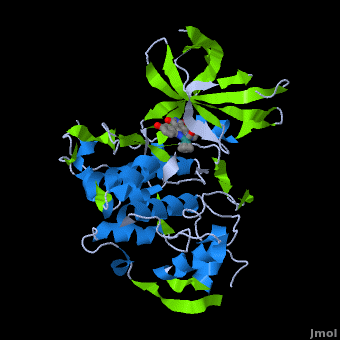
<StructureSection load='' size='350' side='right' scene='Journal:JBIC:2/Opening/1' caption='Crystal Structure of Glycogen Synthase Kinase 3ß bound to Anticancer Ruthenium Complex (PDB code [[3m1s]])'> | <StructureSection load='' size='350' side='right' scene='Journal:JBIC:2/Opening/1' caption='Crystal Structure of Glycogen Synthase Kinase 3ß bound to Anticancer Ruthenium Complex (PDB code [[3m1s]])'> | ||
| - | '''Glycogen synthase kinase 3''' (GSK-3) is a serine/threonine protein kinase. GSK-3 is active in a number of intracellular signaling pathways. GSK-3 regulates glycogen synthase as well as other proteins | + | == Function == |
| + | '''Glycogen synthase kinase 3''' (GSK-3) is a serine/threonine protein kinase. GSK-3 is active in a number of intracellular signaling pathways. GSK-3 regulates glycogen synthase as well as other proteins. For more details see [[Student Project 9 for UMass Chemistry 423 Spring 2015]]. | ||
| - | + | == Relevance == | |
| + | GSK-3 inhibition is studied as a therapeutic target in diseases like Alzheimer, diabetes, bipolar disorder and some cancers. | ||
| + | |||
| + | == Structure of Anticancer Ruthenium Half-Sandwich Complex Bound to Glycogen Synthase Kinase 3ß <ref>DOI 10.1007/s00775-010-0699-x</ref> == | ||
A crystal structure of an <scene name='Journal:JBIC:2/Half_sandwich_complex_no_bonds/1'>organometallic half-sandwich ruthenium complex </scene>bound to the protein kinase glycogen synthase kinase 3ß (GSK-3ß) has been determined and reveals that the inhibitor binds to the <scene name='Journal:JBIC:2/Atp_binding_site2/2'>ATP binding site</scene> via an induced fit mechanism utlizing several <scene name='Journal:JBIC:2/Half_sandwich_complex/3'>hydrogen bonds</scene> and <scene name='Journal:JBIC:2/Half_sandwich_hydrophobic_stic/1'>hydrophobic interactions</scene>. Importantly, the metal is not involved in any direct interaction with the protein kinase but fulfills a purely structural role. The unique, bulky molecular structure of the half-sandwich complex with the CO-ligand oriented perpendicular to the pyridocarbazole heterocycle allows the complex to stretch the whole distance <scene name='Journal:JBIC:2/Half_sandwich_hydrophobic/5'>sandwiched between the faces of the N- and C-terminal lobes</scene> and to interact tightly with <scene name='Journal:JBIC:2/Glycine_rich_loop2/4'>the flexible glycine-rich loop</scene>. Although this complex is a conventional ATP-competitive binder, the unique shape of the complex allows novel interactions with the glycine-rich loop which are crucial for binding potency and selectivity. It can be hypothesized that coordination spheres which present other ligands towards the glycine-rich loop might display completely different protein kinase selectivities. | A crystal structure of an <scene name='Journal:JBIC:2/Half_sandwich_complex_no_bonds/1'>organometallic half-sandwich ruthenium complex </scene>bound to the protein kinase glycogen synthase kinase 3ß (GSK-3ß) has been determined and reveals that the inhibitor binds to the <scene name='Journal:JBIC:2/Atp_binding_site2/2'>ATP binding site</scene> via an induced fit mechanism utlizing several <scene name='Journal:JBIC:2/Half_sandwich_complex/3'>hydrogen bonds</scene> and <scene name='Journal:JBIC:2/Half_sandwich_hydrophobic_stic/1'>hydrophobic interactions</scene>. Importantly, the metal is not involved in any direct interaction with the protein kinase but fulfills a purely structural role. The unique, bulky molecular structure of the half-sandwich complex with the CO-ligand oriented perpendicular to the pyridocarbazole heterocycle allows the complex to stretch the whole distance <scene name='Journal:JBIC:2/Half_sandwich_hydrophobic/5'>sandwiched between the faces of the N- and C-terminal lobes</scene> and to interact tightly with <scene name='Journal:JBIC:2/Glycine_rich_loop2/4'>the flexible glycine-rich loop</scene>. Although this complex is a conventional ATP-competitive binder, the unique shape of the complex allows novel interactions with the glycine-rich loop which are crucial for binding potency and selectivity. It can be hypothesized that coordination spheres which present other ligands towards the glycine-rich loop might display completely different protein kinase selectivities. | ||
</StructureSection> | </StructureSection> | ||
Revision as of 08:54, 14 March 2016
| |||||||||||
3D structures of glycogen synthase kinase 3
Updated on 14-March-2016
References
- ↑ Atilla-Gokcumen GE, Di Costanzo L, Meggers E. Structure of anticancer ruthenium half-sandwich complex bound to glycogen synthase kinase 3beta. J Biol Inorg Chem. 2010 Sep 7. PMID:20821241 doi:10.1007/s00775-010-0699-x