We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Glycogenin
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
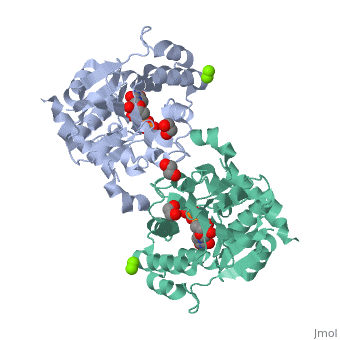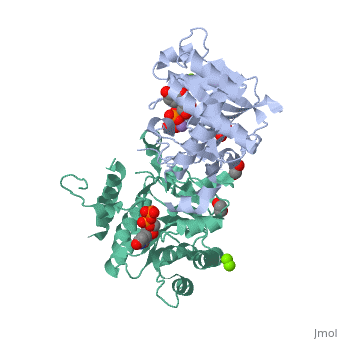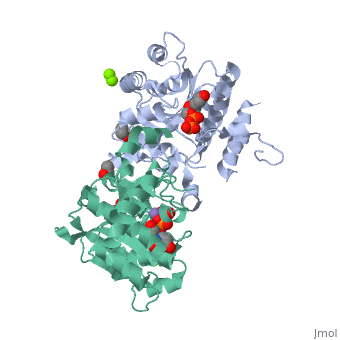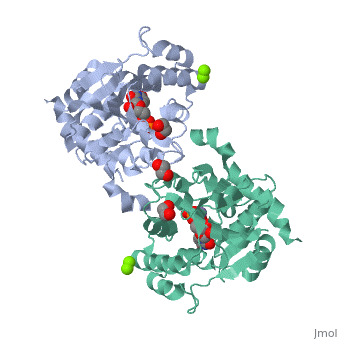
<StructureSection load='3rmv' size='450' side='right' scene='' caption='Human glycogenin-1 complex with UDP (stick model), ethanediol, Mg+2 (green) and Mn+2 (purple) ions (PDB code [[3rmv]])'> | <StructureSection load='3rmv' size='450' side='right' scene='' caption='Human glycogenin-1 complex with UDP (stick model), ethanediol, Mg+2 (green) and Mn+2 (purple) ions (PDB code [[3rmv]])'> | ||
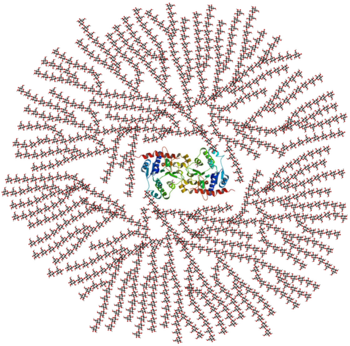
[[Image:Glycogen_structure.png|thumb|left|350x350px|alt=Alt text|Figure 1. A cross-sectional view of glycogen with the glycogenin dimer remaining covalently attached to the non-reducing end in the centre of the globule.]] | [[Image:Glycogen_structure.png|thumb|left|350x350px|alt=Alt text|Figure 1. A cross-sectional view of glycogen with the glycogenin dimer remaining covalently attached to the non-reducing end in the centre of the globule.]] | ||
| + | ==Introduction== | ||
| + | |||
'''Glycogenin''' (Glycogenin glucosyltransferase, [[EC]] 2.4.1.186) is a [[transferase]] responsible for the biosynthesis of glycogen; an important storage form of glucose in the body. It is a unique enzyme in that it is primer, substrate, catalyst, and product of its enzymatic reaction and extension process of glycogen biosynthesis. This is initiated by its ability to transfer glucose from UDP-glucose to form an oligosaccharide of glucose units that is covalently attached to itself at Tyr-194 in a multistep reaction mechanism <ref name="one"> PMID:12051921 </ref>. It is placed in glycosyltransferase family 8 becuase it contains highly conserved motifs that are common to glycosyltransferases such as lipopolysaccharide glucose and galactose transferases and galactinol synthases <ref name="two"> PMID:9345621 </ref>. | '''Glycogenin''' (Glycogenin glucosyltransferase, [[EC]] 2.4.1.186) is a [[transferase]] responsible for the biosynthesis of glycogen; an important storage form of glucose in the body. It is a unique enzyme in that it is primer, substrate, catalyst, and product of its enzymatic reaction and extension process of glycogen biosynthesis. This is initiated by its ability to transfer glucose from UDP-glucose to form an oligosaccharide of glucose units that is covalently attached to itself at Tyr-194 in a multistep reaction mechanism <ref name="one"> PMID:12051921 </ref>. It is placed in glycosyltransferase family 8 becuase it contains highly conserved motifs that are common to glycosyltransferases such as lipopolysaccharide glucose and galactose transferases and galactinol synthases <ref name="two"> PMID:9345621 </ref>. | ||
| - | __TOC__ | ||
==Structure Overview== | ==Structure Overview== | ||
Revision as of 09:07, 14 March 2016
| |||||||||||
3D structures of glycogenin
Updated on 14-March-2016
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Gibbons BJ, Roach PJ, Hurley TD. Crystal structure of the autocatalytic initiator of glycogen biosynthesis, glycogenin. J Mol Biol. 2002 May 31;319(2):463-77. PMID:12051921 doi:http://dx.doi.org/10.1016/S0022-2836(02)00305-4
- ↑ Henrissat B, Davies G. Structural and sequence-based classification of glycoside hydrolases. Curr Opin Struct Biol. 1997 Oct;7(5):637-44. PMID:9345621
- ↑ Alonso MD, Lomako J, Lomako WM, Whelan WJ. A new look at the biogenesis of glycogen. FASEB J. 1995 Sep;9(12):1126-37. PMID:7672505
- ↑ 4.0 4.1 Shearer J, Wilson RJ, Battram DS, Richter EA, Robinson DL, Bakovic M, Graham TE. Increases in glycogenin and glycogenin mRNA accompany glycogen resynthesis in human skeletal muscle. Am J Physiol Endocrinol Metab. 2005 Sep;289(3):E508-14. Epub 2005 May 3. PMID:15870102 doi:10.1152/ajpendo.00100.2005
- ↑ 5.0 5.1 Moslemi AR, Lindberg C, Nilsson J, Tajsharghi H, Andersson B, Oldfors A. Glycogenin-1 deficiency and inactivated priming of glycogen synthesis. N Engl J Med. 2010 Apr 1;362(13):1203-10. PMID:20357282 doi:10.1056/NEJMoa0900661
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, David Canner, Kim Settle