User:Daniel Schemenauer/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
== Introduction == | == Introduction == | ||
G-coupled protein receptors [https://en.wikipedia.org/wiki/G_protein–coupled_receptor GPCR] are trans-membrane proteins that are integral to cell signaling. The human genome encodes for approximately 750 GPCRS, 350 of which are known to respond to extracellular ligands.<ref name="GPCRRep">PMID: 12679517 </ref>. GPCRs are divided into four major classes based on sequence similarity and transduction mechanism; Class A,B,C, and F.<ref name="MSGPCR">PMID:23407534</ref>. Metabotropic Glutamate Receptor 5 (mGlu<sub>5</sub>) is a class C GPCR that is involved in the G<sub>q</sub> pathway <ref name="CCGPCR">PMID:12782243</ref>. mGlu<sub>5</sub>is highly expressed in neuronal and glial cells in the central nervous system, where glutamate serves as the major neurotransmitter. When glutamate binds to the extracellular domain of mGlu<sub>5</sub>, consisting of the Venus Fly Trap <ref name="Primary">PMID: 25042998 </ref>, a conformational change through the tram-membrane domains activates the coupled G-protein. This G-protein disassociates and the alpha subunit activates [https://en.wikipedia.org/wiki/Phospholipase_C Phospholipase C] which has the final outcome of increased neuronal activity<ref name="MSGPCR">PMID:23407534</ref>. | G-coupled protein receptors [https://en.wikipedia.org/wiki/G_protein–coupled_receptor GPCR] are trans-membrane proteins that are integral to cell signaling. The human genome encodes for approximately 750 GPCRS, 350 of which are known to respond to extracellular ligands.<ref name="GPCRRep">PMID: 12679517 </ref>. GPCRs are divided into four major classes based on sequence similarity and transduction mechanism; Class A,B,C, and F.<ref name="MSGPCR">PMID:23407534</ref>. Metabotropic Glutamate Receptor 5 (mGlu<sub>5</sub>) is a class C GPCR that is involved in the G<sub>q</sub> pathway <ref name="CCGPCR">PMID:12782243</ref>. mGlu<sub>5</sub>is highly expressed in neuronal and glial cells in the central nervous system, where glutamate serves as the major neurotransmitter. When glutamate binds to the extracellular domain of mGlu<sub>5</sub>, consisting of the Venus Fly Trap <ref name="Primary">PMID: 25042998 </ref>, a conformational change through the tram-membrane domains activates the coupled G-protein. This G-protein disassociates and the alpha subunit activates [https://en.wikipedia.org/wiki/Phospholipase_C Phospholipase C] which has the final outcome of increased neuronal activity<ref name="MSGPCR">PMID:23407534</ref>. | ||
| - | |||
| Line 11: | Line 10: | ||
===Key Interactions=== | ===Key Interactions=== | ||
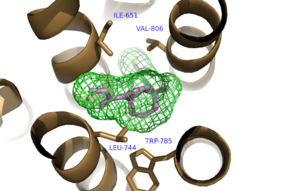
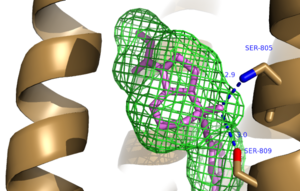
A number of intramolecular interactions within the trans-membrane domain stabilize the inactive conformation of mGlu<sub>5</sub>. The first of these interactions is an ionic interaction, termed the <scene name='72/726409/Ionic_lock2/1'>Ionic Lock </scene>, between Lysine 665 of TM3 and Glutamate 770 of TM6. Evidence for the importance of this interaction came through a kinetic study of mutant proteins where both residues were separately mutated to alanine resulting in constitutive activity of the GPCR and its coupled pathway<ref name="Primary">PMID: 25042998 </ref>.A second critical interaction that stabilizes the inactive conformer is a <scene name='72/726409/Hydrogen_bond_614-668/1'>Hydrogen Bond </scene> between Serine 614 of ICL1 and Arginine 668 of TM3. Similarly, when Serine 614 is mutated to alanine high levels of activity are seen in the mutant GPCR<ref name="Primary">PMID: 25042998 </ref>. | A number of intramolecular interactions within the trans-membrane domain stabilize the inactive conformation of mGlu<sub>5</sub>. The first of these interactions is an ionic interaction, termed the <scene name='72/726409/Ionic_lock2/1'>Ionic Lock </scene>, between Lysine 665 of TM3 and Glutamate 770 of TM6. Evidence for the importance of this interaction came through a kinetic study of mutant proteins where both residues were separately mutated to alanine resulting in constitutive activity of the GPCR and its coupled pathway<ref name="Primary">PMID: 25042998 </ref>.A second critical interaction that stabilizes the inactive conformer is a <scene name='72/726409/Hydrogen_bond_614-668/1'>Hydrogen Bond </scene> between Serine 614 of ICL1 and Arginine 668 of TM3. Similarly, when Serine 614 is mutated to alanine high levels of activity are seen in the mutant GPCR<ref name="Primary">PMID: 25042998 </ref>. | ||
| - | + | A <scene name='72/726404/Scene_6/3'>Disulfide Bond </scene> between Cystine 644 of TM3 and Cystine 733 of ECL2 is critical at anchoring ECL2 and is highly conserved across Class C GPCR’s<ref name="Primary">PMID: 25042998 </ref>. The ECL2 position, in combination with the helical bundle of the trans-membrane domain, create a <scene name='72/726409/Electrogradient2/3'>Binding Cap</scene> that restricts entrance to the allosteric binding site within the seven trans-membrane α-helices. This restricted entrance has no effect on the natural ligand, glutamate, as it binds to the extracellular domain, but dictates potential drug targets that act through allosteric modulation<ref name="Primary">PMID: 25042998 </ref>. | |
| - | + | ||
| - | + | ||
| - | <scene name='72/726404/Scene_6/3'>Disulfide | + | |
| - | <scene name='72/726409/Electrogradient2/3'> | + | |
| - | + | ||
== Clinical Relevance == | == Clinical Relevance == | ||
Revision as of 15:14, 28 March 2016
| |||||||||||
References
- ↑ Vassilatis DK, Hohmann JG, Zeng H, Li F, Ranchalis JE, Mortrud MT, Brown A, Rodriguez SS, Weller JR, Wright AC, Bergmann JE, Gaitanaris GA. The G protein-coupled receptor repertoires of human and mouse. Proc Natl Acad Sci U S A. 2003 Apr 15;100(8):4903-8. Epub 2003 Apr 4. PMID:12679517 doi:http://dx.doi.org/10.1073/pnas.0230374100
- ↑ 2.0 2.1 Venkatakrishnan AJ, Deupi X, Lebon G, Tate CG, Schertler GF, Babu MM. Molecular signatures of G-protein-coupled receptors. Nature. 2013 Feb 14;494(7436):185-94. doi: 10.1038/nature11896. PMID:23407534 doi:http://dx.doi.org/10.1038/nature11896
- ↑ Pin JP, Galvez T, Prezeau L. Evolution, structure, and activation mechanism of family 3/C G-protein-coupled receptors. Pharmacol Ther. 2003 Jun;98(3):325-54. PMID:12782243
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 Dore AS, Okrasa K, Patel JC, Serrano-Vega M, Bennett K, Cooke RM, Errey JC, Jazayeri A, Khan S, Tehan B, Weir M, Wiggin GR, Marshall FH. Structure of class C GPCR metabotropic glutamate receptor 5 transmembrane domain. Nature. 2014 Jul 31;511(7511):557-62. doi: 10.1038/nature13396. Epub 2014 Jul 6. PMID:25042998 doi:http://dx.doi.org/10.1038/nature13396