We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1170
From Proteopedia
(Difference between revisions)
| Line 13: | Line 13: | ||
=== Charge Network === | === Charge Network === | ||
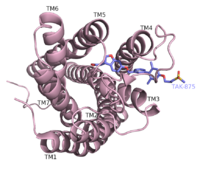
| - | hGPR40 has a distinct binding pocket that is established by seven key residues.[[Image:charge network residues.png|300 px|right|thumb| | + | hGPR40 has a distinct binding pocket that is established by seven key residues.[[Image:charge network residues.png|300 px|right|thumb|TAK-875 binding residues]] |
The importance of these residues for agonist binding was determined by mutagenesis studies. Each of these residues have either a charged or polar R-group that allows them to develop a charge network. This network keeps the residues in a stable, unbound state until exposed to a substrate. When the substrate (an agonist) enters the binding pocket, four of the seven <scene name='72/721541/Hydrogen_binding_1/3'>Key Binding Residues</scene> interact directly with the carboxylate moiety of the agonist. In 2007 and 2009 researchers showed the presence of Arg 183 and Arg 258 in the binding pocket <ref name="Sum">PMID: 17699519</ref><ref name="Sum, C.">PMID:19068482</ref> Along with the two Arginine residues, the charge network incorporates two Tyrosine residues.These residues (Tyr 91 and Tyr 240) also stabilize the carboxylate of the agonists. It was further determined that Tyr 240 is epecially important for binding. Mutation of Tyr 240 caused a reduction in the binding affinity of TAK-875 by eight fold and had a significant effect on the Kd of the protein.<ref name="Srivastava"/> | The importance of these residues for agonist binding was determined by mutagenesis studies. Each of these residues have either a charged or polar R-group that allows them to develop a charge network. This network keeps the residues in a stable, unbound state until exposed to a substrate. When the substrate (an agonist) enters the binding pocket, four of the seven <scene name='72/721541/Hydrogen_binding_1/3'>Key Binding Residues</scene> interact directly with the carboxylate moiety of the agonist. In 2007 and 2009 researchers showed the presence of Arg 183 and Arg 258 in the binding pocket <ref name="Sum">PMID: 17699519</ref><ref name="Sum, C.">PMID:19068482</ref> Along with the two Arginine residues, the charge network incorporates two Tyrosine residues.These residues (Tyr 91 and Tyr 240) also stabilize the carboxylate of the agonists. It was further determined that Tyr 240 is epecially important for binding. Mutation of Tyr 240 caused a reduction in the binding affinity of TAK-875 by eight fold and had a significant effect on the Kd of the protein.<ref name="Srivastava"/> | ||
Revision as of 12:24, 29 March 2016
Human GPR40, also known as Free Fatty Acid Receptor 1 (FFAR1)
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 Srivastava A, Yano J, Hirozane Y, Kefala G, Gruswitz F, Snell G, Lane W, Ivetac A, Aertgeerts K, Nguyen J, Jennings A, Okada K. High-resolution structure of the human GPR40 receptor bound to allosteric agonist TAK-875. Nature. 2014 Jul 20. doi: 10.1038/nature13494. PMID:25043059 doi:http://dx.doi.org/10.1038/nature13494
- ↑ Kebede M, Ferdaoussi M, Mancini A, Alquier T, Kulkarni RN, Walker MD, Poitout V. Glucose activates free fatty acid receptor 1 gene transcription via phosphatidylinositol-3-kinase-dependent O-GlcNAcylation of pancreas-duodenum homeobox-1. Proc Natl Acad Sci U S A. 2012 Feb 14;109(7):2376-81. doi:, 10.1073/pnas.1114350109. Epub 2012 Jan 30. PMID:22308370 doi:http://dx.doi.org/10.1073/pnas.1114350109
- ↑ Ma Z, Lin DC, Sharma R, Liu J, Zhu L, Li AR, Kohn T, Wang Y, Liu JJ, Bartberger MD, Medina JC, Zhuang R, Li F, Zhang J, Luo J, Wong S, Tonn GR, Houze JB. Discovery of the imidazole-derived GPR40 agonist AM-3189. Bioorg Med Chem Lett. 2016 Jan 1;26(1):15-20. doi: 10.1016/j.bmcl.2015.11.050., Epub 2015 Nov 17. PMID:26620255 doi:http://dx.doi.org/10.1016/j.bmcl.2015.11.050
- ↑ 4.0 4.1 4.2 4.3 Burant CF. Activation of GPR40 as a therapeutic target for the treatment of type 2 diabetes. Diabetes Care. 2013 Aug;36 Suppl 2:S175-9. doi: 10.2337/dcS13-2037. PMID:23882043 doi:http://dx.doi.org/10.2337/dcS13-2037
- ↑ Sum CS, Tikhonova IG, Neumann S, Engel S, Raaka BM, Costanzi S, Gershengorn MC. Identification of residues important for agonist recognition and activation in GPR40. J Biol Chem. 2007 Oct 5;282(40):29248-55. Epub 2007 Aug 15. PMID:17699519 doi:http://dx.doi.org/10.1074/jbc.M705077200
- ↑ Sum CS, Tikhonova IG, Costanzi S, Gershengorn MC. Two arginine-glutamate ionic locks near the extracellular surface of FFAR1 gate receptor activation. J Biol Chem. 2009 Feb 6;284(6):3529-36. doi: 10.1074/jbc.M806987200. Epub 2008, Dec 8. PMID:19068482 doi:http://dx.doi.org/10.1074/jbc.M806987200
- ↑ Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, Tanaka H, Maruyama M, Satoh R, Okubo S, Kizawa H, Komatsu H, Matsumura F, Noguchi Y, Shinohara T, Hinuma S, Fujisawa Y, Fujino M. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 2003 Mar 13;422(6928):173-6. Epub 2003 Feb 23. PMID:12629551 doi:http://dx.doi.org/10.1038/nature01478