We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1160
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
The mGlu family of receptors was the first of the Class C GPCR to be extensively studied<ref name="Wu" />. The first regions of the protein crystallized and studied were the Venus fly trap domain and the cystiene-rich domain on the extracellular region of the receptor<ref name="Dore" />. The hydrophobic nature and flexibility of the transmembrane domain made it difficult to crystallize. Recently, the human metabotropic glutamate receptor 5 transmembrane domain (TMD) was crystallized and a structure elucidated<ref name="Dore" />. There were several necessary modifications made to the TMD for it to successfully crystallize. The protein was thermostabilized and flexible domains were removed<ref name="Dore" />. In total residue 2-568 and residues 837-1153 were excised from the structure. Also, a T4 -<scene name='72/721531/Protien_lys/1'>Lysozyme</scene> was inserted into ICL-2 to add stability<ref name="Dore" />. | The mGlu family of receptors was the first of the Class C GPCR to be extensively studied<ref name="Wu" />. The first regions of the protein crystallized and studied were the Venus fly trap domain and the cystiene-rich domain on the extracellular region of the receptor<ref name="Dore" />. The hydrophobic nature and flexibility of the transmembrane domain made it difficult to crystallize. Recently, the human metabotropic glutamate receptor 5 transmembrane domain (TMD) was crystallized and a structure elucidated<ref name="Dore" />. There were several necessary modifications made to the TMD for it to successfully crystallize. The protein was thermostabilized and flexible domains were removed<ref name="Dore" />. In total residue 2-568 and residues 837-1153 were excised from the structure. Also, a T4 -<scene name='72/721531/Protien_lys/1'>Lysozyme</scene> was inserted into ICL-2 to add stability<ref name="Dore" />. | ||
== Structure== | == Structure== | ||
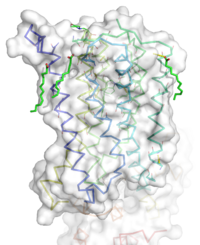
| - | [[Image:STR.png|200 px|left|thumb|Overall Structure of the TMD. The polar heads on the Oliec acids orient the protein with the top of the image being the extracellular portion of the protein,the middle portion inserted into the membrane, and the lower portion located inside of the cell. ]] | + | [[Image:STR.png|200 px|left|thumb|Overall Structure of the TMD. The polar heads on the Oliec acids orient the protein with the top of the image being the extracellular portion of the protein, the middle portion inserted into the membrane, and the lower portion located inside of the cell. ]] |
=== Overview === | === Overview === | ||
| - | The mGlu5 TMD contains 7 <scene name='72/721531/Protien_7_helices/2'> alpha helices</scene> that span the membrane. The protein was crystallized with Oleic acid and MES. On the superior portion of the protein there are several critical extracellular loops.The binding pocket can be found near the middle of the protein.Inserted into the biding pocket is the negative allosteric modulator [http://www.en.wikipedia.org/wiki/Mavoglurant mavoglurant]. It is important to note that the TMD as illustrated is in an inactive conformation. On the intracellular portion of the protein there exist several ionic locks whose positions will determine the activity of the protein. | + | The mGlu5 TMD contains 7 <scene name='72/721531/Protien_7_helices/2'> alpha helices</scene> that span the membrane. The protein was crystallized with Oleic acid and MES. On the superior portion of the protein there are several critical extracellular loops. The binding pocket can be found near the middle of the protein. Inserted into the biding pocket is the negative allosteric modulator [http://www.en.wikipedia.org/wiki/Mavoglurant mavoglurant]. It is important to note that the TMD as illustrated is in an inactive conformation. On the intracellular portion of the protein there exist several ionic locks whose positions will determine the activity of the protein. |
=== Extracellular Domain === | === Extracellular Domain === | ||
| - | These are the <scene name='72/721532/Ecl_trail_1/7'>Extracellular Loops</scene> with extracellular loops (ECLs) 1, 2, and 3 highlighted in purple. Additionally in the ECL domain, a <scene name='72/721532/Ecl_trail_1/6'>disulfide bond</scene> is attached to both Helix 3 and the amino acid chain between Helix 5 and the N terminus. The disulfide bond is highlighted in yellow, and it is conserved in all classes of glutamate receptor 5 transmembrane domains<ref name="Wu" />. | + | These are the <scene name='72/721532/Ecl_trail_1/7'>Extracellular Loops</scene> with extracellular loops (ECLs) 1, 2, and 3 highlighted in purple. Additionally in the ECL domain, a <scene name='72/721532/Ecl_trail_1/6'>disulfide bond</scene> is attached to both Helix 3 and the amino acid chain between Helix 5 and the N terminus. The disulfide bond is highlighted in yellow, and it is conserved in all classes of glutamate receptor 5 transmembrane domains<ref name="Wu" />. |
=== Binding Pocket === | === Binding Pocket === | ||
[[Image: Organic with clipped surface.png|200 px|left|thumb|Cross section view of mavoglurant in the binding pocket]] | [[Image: Organic with clipped surface.png|200 px|left|thumb|Cross section view of mavoglurant in the binding pocket]] | ||
| Line 21: | Line 21: | ||
*A <scene name='72/721531/Protien_bindbottom/1'>water molecular</scene> inside of the binding pocket helps stabilize the inactive state. | *A <scene name='72/721531/Protien_bindbottom/1'>water molecular</scene> inside of the binding pocket helps stabilize the inactive state. | ||
| - | Once bound to mavoglurant, transmembrane helix 7 undergoes a conformational change<ref name="Dore" />. The shifting of TM7 will lead to a more global conformational change, which we leave the receptor incapable of signaling<ref name="Dore" />.Variation can be seen in positioning of alpha helices. Class C receptors have seemingly less space for [https://fragilex.org/2014/research/news-reports-and-commentaries/novartis-announces-results-of-mavoglurant-mglur5-afq056-clinical-trials-and-the-conclusion-of-the-long-term-extension-study/ mavoglurant] to enter as compared to Class A and F receptors<ref name="Wu" />. The ligand binding site is also varied between different classes of mGlu receptors<ref name="Dore" />. | + | Once bound to mavoglurant, transmembrane helix 7 undergoes a conformational change<ref name="Dore" />. The shifting of TM7 will lead to a more global conformational change, which we leave the receptor incapable of signaling<ref name="Dore" />. Variation can be seen in positioning of alpha helices across receptor class. Class C receptors have seemingly less space for [https://fragilex.org/2014/research/news-reports-and-commentaries/novartis-announces-results-of-mavoglurant-mglur5-afq056-clinical-trials-and-the-conclusion-of-the-long-term-extension-study/ mavoglurant] to enter as compared to Class A and F receptors<ref name="Wu" />. The position of the ligand binding site is also varied between different classes of mGlu receptors<ref name="Dore" />. |
=== Ionic Locks === | === Ionic Locks === | ||
| - | Another important structural feature | + | Another important structural feature is the series of <scene name='72/721531/Ionic_lock/2'>ionic locks</scene> on the intracellular side of the protein. Interactions between amino acids will form a salt bridge, which will stabilize the inactive conformation<ref name="Dore" />. The primary ionic lock forms between Glu770, Lys665, and Ser613<ref name="Dore" />. A secondary ionic lock occurs between Ser614 and Arg668<ref name="Dore" />. The purpose of these ionic locks is analogous to the ionic interactions that stabilize the T state in [[Hemoglobin]]. In the case of the TMD, when the NAM mavoglurant is bound the ionic lock is formed. This stabilizes the inactive state, where the intracellular loops are stabilized inwards<ref name="Wu" />. This will effectively block the crevice that is involved in binding the G-protein<ref name="Wu" />. Models have suggested that, even in a glutamate bound state, the mavoglurant bound receptor would be dimerized but incapable of signaling<ref name="Wu" />. This helps maintain the readiness of the pathway, while still decreasing signal response. |
| - | == | + | == Pathway == |
| - | It all begins with glutamate binding to the Venus flytrap domain. The signal | + | It all begins with glutamate binding to the Venus flytrap domain. The signal is transduced across the cystine-rich domain to the TMD. Next, the dimerization of the TMD occurs. This activates the Gq/11 pathway, which activates phspholipase Cβ<ref name="Niswender" />. The active phospholipase Cβ hydrolyzes phosphotinositides and generates inositol 1,4,5-trisphosphate and diacyl-glycerol<ref name="Woodcock" />. This results in calcium mobilization and activation of PKC<ref name="Niswender" />. |
== Disease == | == Disease == | ||
=== Fragile X === | === Fragile X === | ||
| - | Fragile X syndrome is the most common genetic cause of mental | + | Fragile X syndrome is the most common genetic cause of mental disability, and is a member of the Autism spectrum disorder family<ref name="Bailey" />. The severity of intellectual disability can vary from patient to patient, but symptoms stem from a misregulation of the mGlu1 and MGlu5 pathways<ref name="Bailey" />. This leads to over potentiation in neural cells. Mavoglurant and other allosteric regulators like fenobam have shown promise in treating Fragile X. One positive characteristic of ligands that target the TMD is they tend to be more specific, thus interacting less with brain proteins<ref name="Feng" />. Mavoglurant would act to down regulate glutamate signaling in an attempt to decrease potentiation. Unfortunately, recent Phase 2 clinical trials have proven mavoglurant ineffective <ref name="Bailey" />. Novartis the company who developed the drug has stopped clinical trials of mavoglurant <ref name="Bailey" />. However, modulators of mGlu5 TMD are still being researched to treat Parkinson's, Alzheimer's disease, and various addictions<ref name="Niswender" />. |
Revision as of 12:35, 30 March 2016
Human metabotropic glutamate receptor 5 transmembrane domain
| |||||||||||