User:Brittany Stankavich/Sandbox 1
From Proteopedia
| Line 26: | Line 26: | ||
=== Ligand Binding === | === Ligand Binding === | ||
| + | |||
| + | GPR40’s natural substrate are FFAs in which a free carboxyl group is required to bind. However, GPR40 can be activated by a wide variety of fatty acids with chain lengths ranging from saturated fatty acids with 8 carbons to 23 carbons. In addition, various mono (i.e. palmitoleic (C16:1) and oleic (C18:1) acids) and poly-unsaturated fatty acids (i.e. linoleic (C18:2) and eicosatrienoic (C20:3) acids) can activate GPR40 (Morgan et al. 2009). The agonists potency varies according to the carbon-chain length however. The activity of GPR40 increases when the chain is increased from C6 to C15 but then decreased when the chain was extended beyond C15. One explanation for this is that as alkyl chain increased, so did the hydrophobic interactions with the protein within the binding pocket. However, for FFAs with carbon chains longer than C15, the molecular size is too large for the binding pocket. This causes the alkyl chain to extend beyond the binding pocket and destabilize the binding (Ren et al. 2016). | ||
| + | |||
| + | |||
<scene name='72/727085/Hgpr40_binding_relay/5'>TAK-875</scene> | <scene name='72/727085/Hgpr40_binding_relay/5'>TAK-875</scene> | ||
Revision as of 15:25, 31 March 2016
- User:Brittany Stankavich/Sandbox 1
Contents |
hGPR40 Homo sapiens
|
Introduction
Function
Signal Transduction
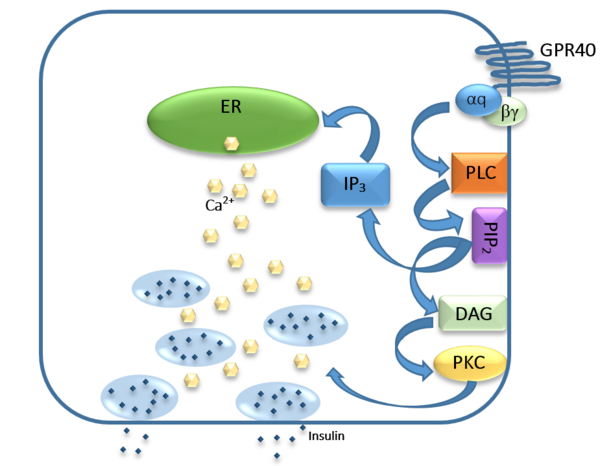
FFAs bind to GPR40 which then couples with the G-protein Gq leading to increased phospholipase C (PLC) activity. PLC catalyzes the hydrolysis of the phospholipid phosphatidylinositol-4,5-biphosphate (PIP2) resulting in the formation of diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3). DAG can activate protein kinase C (PKC) to enhance insulin secretion. IP3 on the other hand is soluble and diffuses to the endoplasmic reticulum where it is able to bind to a receptor on a ligand-gated Ca2+ channel. This binding triggers the opening of the channel causing stored Ca2+ to be released into the cytoplasm. Upon this large increase in intracellular free Ca2+, there is also an increase in glucose-dependent insulin secretion suggesting that insulin release can be contributed in part to the changes in Ca2+ concentration resulting from activated GPR40.
Structure
Ligand Binding
GPR40’s natural substrate are FFAs in which a free carboxyl group is required to bind. However, GPR40 can be activated by a wide variety of fatty acids with chain lengths ranging from saturated fatty acids with 8 carbons to 23 carbons. In addition, various mono (i.e. palmitoleic (C16:1) and oleic (C18:1) acids) and poly-unsaturated fatty acids (i.e. linoleic (C18:2) and eicosatrienoic (C20:3) acids) can activate GPR40 (Morgan et al. 2009). The agonists potency varies according to the carbon-chain length however. The activity of GPR40 increases when the chain is increased from C6 to C15 but then decreased when the chain was extended beyond C15. One explanation for this is that as alkyl chain increased, so did the hydrophobic interactions with the protein within the binding pocket. However, for FFAs with carbon chains longer than C15, the molecular size is too large for the binding pocket. This causes the alkyl chain to extend beyond the binding pocket and destabilize the binding (Ren et al. 2016).
The carboxylic acid terminus of TAK-875 is buried within a very hydrophobic region where it interacts with polar residues Arg 183, Arg 258, Tyr 91, and Tyr 240.
Binding Pockets
Medical Relevance
Applications
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
</StructureSection>