User:Brittany Stankavich/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 38: | Line 38: | ||
The carboxylic acid terminus of TAK-875 is buried within a very hydrophobic region where it interacts with polar residues Arg 183, Arg 258, Tyr 91, and Tyr 240. | The carboxylic acid terminus of TAK-875 is buried within a very hydrophobic region where it interacts with polar residues Arg 183, Arg 258, Tyr 91, and Tyr 240. | ||
| + | |||
| Line 47: | Line 48: | ||
== Medical Relevance == | == Medical Relevance == | ||
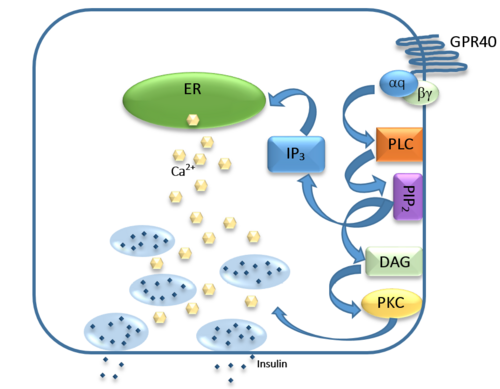
| - | While undergoing clinical trials, the use of TAK-875 in the treatment of diabetes mellitus was terminated in the III phase. This was due to observed liver toxicity. The liver toxicity is believed to be due to its effects on the [https://en.wikipedia.org/wiki/Bile_acid bile acids], achieved through the inhibition of the efflux of bile acids into bile <ref>PMID:26276582</ref>. Other molecules are currently undergoing development in an effort to activate hGPR40 in a way that does not adversely affect the liver. The leading research suggests a molecule similar to that of 3-ethoxypropanoic acid <ref>PMID:25815144</ref>. However, this molecule must be modified before it could be conceivably used, as its [https://en.wikipedia.org/wiki/Half-life half-life] is extremely short, due to the rapid [https://en.wikipedia.org/wiki/Redox oxidation] at the [https://en.wikipedia.org/wiki/Benzyl benzyl position] during metabolism <ref | + | While undergoing clinical trials, the use of TAK-875 in the treatment of diabetes mellitus was terminated in the III phase. This was due to observed liver toxicity. The liver toxicity is believed to be due to its effects on the [https://en.wikipedia.org/wiki/Bile_acid bile acids], achieved through the inhibition of the efflux of bile acids into bile <ref>PMID:26276582</ref>. Other molecules are currently undergoing development in an effort to activate hGPR40 in a way that does not adversely affect the liver. The leading research suggests a molecule similar to that of 3-ethoxypropanoic acid <ref name="Tak">PMID:25815144</ref>. However, this molecule must be modified before it could be conceivably used, as its [https://en.wikipedia.org/wiki/Half-life half-life] is extremely short, due to the rapid [https://en.wikipedia.org/wiki/Redox oxidation] at the [https://en.wikipedia.org/wiki/Benzyl benzyl position] during metabolism <ref name= "Tak">. Additional research may hold the answer to effective treatment of type 2 diabetes and should be centered on the hGPR40 receptor because of its unique glucose dependent insulin secretion. |
== Applications == | == Applications == | ||
Revision as of 18:48, 1 April 2016
- User:Brittany Stankavich/Sandbox 1
hGPR40 Homo sapiens
| |||||||||||
References
- ↑ Hanson MA, Roth CB, Jo E, Griffith MT, Scott FL, Reinhart G, Desale H, Clemons B, Cahalan SM, Schuerer SC, Sanna MG, Han GW, Kuhn P, Rosen H, Stevens RC. Crystal structure of a lipid G protein-coupled receptor. Science. 2012 Feb 17;335(6070):851-5. PMID:22344443 doi:10.1126/science.1215904
- ↑ Li X, Zhong K, Guo Z, Zhong D, Chen X. Fasiglifam (TAK-875) Inhibits Hepatobiliary Transporters: A Possible Factor Contributing to Fasiglifam-Induced Liver Injury. Drug Metab Dispos. 2015 Nov;43(11):1751-9. doi: 10.1124/dmd.115.064121. Epub 2015, Aug 14. PMID:26276582 doi:http://dx.doi.org/10.1124/dmd.115.064121
- ↑ 3.0 3.1 Takano R, Yoshida M, Inoue M, Honda T, Nakashima R, Matsumoto K, Yano T, Ogata T, Watanabe N, Hirouchi M, Yoneyama T, Ito S, Toda N. Discovery of DS-1558: A Potent and Orally Bioavailable GPR40 Agonist. ACS Med Chem Lett. 2015 Jan 13;6(3):266-70. doi: 10.1021/ml500391n. eCollection, 2015 Mar 12. PMID:25815144 doi:http://dx.doi.org/10.1021/ml500391n