User:Dean Williams/Sandbox 1180
From Proteopedia
| Line 73: | Line 73: | ||
===Structurally Significant GCGR 7TDM Residues=== | ===Structurally Significant GCGR 7TDM Residues=== | ||
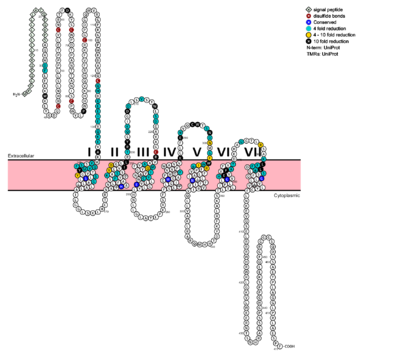
| - | [[Image:Protter GLR HUMAN.png |400 px|left|thumb|Snake Plot of GCGR TMD<ref name= "Siu 2013"/>]] | + | [[Image:Protter GLR HUMAN.png |400 px|left|thumb|Fig. 6: Snake Plot of GCGR TMD<ref name= "Siu 2013"/>]] |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| Line 105: | Line 81: | ||
| + | The snake plot (Fig. 6) shows the conservation and effects of mutagenesis in the 7TMD structure of class B human GPCR. The highly conserved amino acids imply an importance to that functioning of the individual residues and their interactions. The amino acids which have a great impact on the function of the receptor are highlighted in teal, yellow, and black, and offer evidence that the position and interaction of the amino acid is crucial for protein function. Most of the residues that play an important role in glucagon binding face the main cavity of the 7TM structure. Mutagenesis in these positions highly compromises the functioning of the glucagon binding. | ||
| Line 110: | Line 87: | ||
===Peptide binding and selectivity=== | ===Peptide binding and selectivity=== | ||
| - | It has been discovered that the large, soluble N-terminal extracellular domains (ECD) of GCGR are primary in ligand selectivity with the deep, ligand pocket of the TMD providing secondary recognition. <ref name= "Yang 2015"/> [[Image:Movie_Frame_2.png|100 px|left|thumb| | + | It has been discovered that the large, soluble N-terminal extracellular domains (ECD) of GCGR are primary in ligand selectivity with the deep, ligand pocket (Fig. 7) of the TMD providing secondary recognition. <ref name= "Yang 2015"/> [[Image:Movie_Frame_2.png|100 px|left|thumb|Fig. 7: Active site buried deep in 7TMD of glucagon receptor.]] |
===Conformational changes=== | ===Conformational changes=== | ||
| - | Because of the difficulty of stabilizing and crystallizing Class B TMDs, very little is known about the conformational changes that transduce cell signals endogenously. It is known that GCGR can regulate additional signal pathways including G-proteins of the Gαi family through the adoption of differing receptor conformations | + | Because of the difficulty of stabilizing and crystallizing Class B TMDs, very little is known about the conformational changes that transduce cell signals endogenously. It is known that GCGR can regulate additional signal pathways including G-proteins of the Gαi family through the adoption of differing receptor conformations. Research is ongoing. <ref name= "Xu 2009"/> |
| Line 122: | Line 99: | ||
==Kinetics== | ==Kinetics== | ||
GPCR activity is regularly quantified by ligand binding affinity, potency, efficacy, and kinetics. These measurement are used to measure drug ligand interactions in vivo. Recently, GPCRs have been crystallized and catalogued, which tend to include a need to stabilize the receptor, emphasizing the instability of the G coupled protein receptor. Zhang et. al. imply the importance of receptor folding in the cell membrane, in the human class B GPCR the 7TM portion, for receptor stability and function. <ref>DOI 10.1016/j.tibs.2014.12.005</ref> | GPCR activity is regularly quantified by ligand binding affinity, potency, efficacy, and kinetics. These measurement are used to measure drug ligand interactions in vivo. Recently, GPCRs have been crystallized and catalogued, which tend to include a need to stabilize the receptor, emphasizing the instability of the G coupled protein receptor. Zhang et. al. imply the importance of receptor folding in the cell membrane, in the human class B GPCR the 7TM portion, for receptor stability and function. <ref>DOI 10.1016/j.tibs.2014.12.005</ref> | ||
| + | |||
==The Signal Peptide: Glucagon== | ==The Signal Peptide: Glucagon== | ||
| Line 128: | Line 106: | ||
Glucagon, a signaling ligand in the metabolic pathway, has three main biological functions. | Glucagon, a signaling ligand in the metabolic pathway, has three main biological functions. | ||
| - | Glucagon | + | Glucagon is a regulator of the production of cholesterol, which is an energetically intensive process. When energy resources are low, downregulation of cholesterol production begins with glucagon binding to GCGR, which stimulates the phosphorylation of HMG-CoA. Once HMG-CoA has been phosphorylated, it is inactivated and cholesterol production is moderated to conserve energy. |
| + | |||
| + | Glucagon also takes part in fatty acid mobilization by affecting levels of adipose tissue in the organism. Activation of GCGR by glucagon initiates triacylglycerol breakdown and the phosphorylation of perilipin and lipases via cAMP signal pathways. This allows the body to export fatty acids to the liver and other crucial tissues for energy use and makes more glucose available for use in brain functioning. | ||
| - | Glucagon | + | Glucagon is able to increase glucose levels in the blood. Glucagon lowers the concentration of fructose 2,6-bisphosphate which is an allosteric inhibitor of the gluconeogenic enzyme fructose 1,6-bisphosphotase and activates phosphofructose kinase 1, which increases glucose levels via glycolysis. |
<ref name = 'Lehninger'>'Lehninger A., Nelson D.N, & Cox M.M. (2008) Lehninger Principles of Biochemistry. W. H. Freeman, fifth edition.' </ref> | <ref name = 'Lehninger'>'Lehninger A., Nelson D.N, & Cox M.M. (2008) Lehninger Principles of Biochemistry. W. H. Freeman, fifth edition.' </ref> | ||
Revision as of 21:17, 1 April 2016
Structure of Class B Human Glucagon G-Protein Coupled Receptors (GCGRs)
G protein coupled receptors (GPCRs) are recognized as the largest known class of integral membrane proteins and are divided into five families; the rhodopsin family (class A), the secretin family (class B), the adhesion family, the glutamate family (class C), and the frizzled/taste family (class F). Roughly 5% of the human genome encodes g-protein-coupled receptors which are responsible for the transduction of endogenous signals and the instigation of cellular response. The variants all contain a similar seven α-helical transmembrane domain (TMD or 7TMD) that, once bound to its peptide ligand, undergoes conformational change and tranduces a signal to coupled, heterotrimeric G proteins which initiate intracellular signal pathways and generate physiological and pathological processes. [1]
Class B GPCRs contain 15 distinct receptors for peptide hormones and generate their signal pathway through the activation of adenylate cyclase (AC) which increases concentration of cAMP, inositol phosphate, and calcium levels in cyto. [2] These signals are essential elements of intracellular signal cascades for human diseases including type II diabetes mellitus, osteoporosis, obesity, cancer, neurological degeneration, cardiovascular diseases, headaches, and psychiatric disorders; making their regulation through drug targeting of particular interest to companies developing novel molecules. [3] Structurally based approaches to the development of small-molecule agonists and antagonists have been hampered by the lack of accurate Class B TMD visualizations until recent crystal structures of corticoptropin-releasing factor receptor 1 and human glucagon were realized. [4] [5]
The glucagon class B GPCR (GCGR) is involved in glucose homeostasis through the binding of the signal peptide glucagon.
| |||||||||||
References
- ↑ Zhang Y, Devries ME, Skolnick J. Structure modeling of all identified G protein-coupled receptors in the human genome. PLoS Comput Biol. 2006 Feb;2(2):e13. Epub 2006 Feb 17. PMID:16485037 doi:http://dx.doi.org/10.1371/journal.pcbi.0020013
- ↑ Bortolato A, Dore AS, Hollenstein K, Tehan BG, Mason JS, Marshall FH. Structure of Class B GPCRs: new horizons for drug discovery. Br J Pharmacol. 2014 Jul;171(13):3132-45. doi: 10.1111/bph.12689. PMID:24628305 doi:http://dx.doi.org/10.1111/bph.12689
- ↑ 3.0 3.1 Hollenstein K, de Graaf C, Bortolato A, Wang MW, Marshall FH, Stevens RC. Insights into the structure of class B GPCRs. Trends Pharmacol Sci. 2014 Jan;35(1):12-22. doi: 10.1016/j.tips.2013.11.001. Epub, 2013 Dec 18. PMID:24359917 doi:http://dx.doi.org/10.1016/j.tips.2013.11.001
- ↑ Hollenstein K, Kean J, Bortolato A, Cheng RK, Dore AS, Jazayeri A, Cooke RM, Weir M, Marshall FH. Structure of class B GPCR corticotropin-releasing factor receptor 1. Nature. 2013 Jul 25;499(7459):438-43. doi: 10.1038/nature12357. Epub 2013 Jul 17. PMID:23863939 doi:http://dx.doi.org/10.1038/nature12357
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 5.8 Siu FY, He M, de Graaf C, Han GW, Yang D, Zhang Z, Zhou C, Xu Q, Wacker D, Joseph JS, Liu W, Lau J, Cherezov V, Katritch V, Wang MW, Stevens RC. Structure of the human glucagon class B G-protein-coupled receptor. Nature. 2013 Jul 25;499(7459):444-9. doi: 10.1038/nature12393. Epub 2013 Jul 17. PMID:23863937 doi:10.1038/nature12393
- ↑ 6.0 6.1 Yang DH, Zhou CH, Liu Q, Wang MW. Landmark studies on the glucagon subfamily of GPCRs: from small molecule modulators to a crystal structure. Acta Pharmacol Sin. 2015 Sep;36(9):1033-42. doi: 10.1038/aps.2015.78. Epub 2015, Aug 17. PMID:26279155 doi:http://dx.doi.org/10.1038/aps.2015.78
- ↑ Ahren B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov. 2009 May;8(5):369-85. doi: 10.1038/nrd2782. Epub 2009 Apr, 14. PMID:19365392 doi:http://dx.doi.org/10.1038/nrd2782
- ↑ 8.0 8.1 Xu Y, Xie X. Glucagon receptor mediates calcium signaling by coupling to G alpha q/11 and G alpha i/o in HEK293 cells. J Recept Signal Transduct Res. 2009 Dec;29(6):318-25. doi:, 10.3109/10799890903295150. PMID:19903011 doi:http://dx.doi.org/10.3109/10799890903295150
- ↑ Weston C, Lu J, Li N, Barkan K, Richards GO, Roberts DJ, Skerry TM, Poyner D, Pardamwar M, Reynolds CA, Dowell SJ, Willars GB, Ladds G. Modulation of Glucagon Receptor Pharmacology by Receptor Activity-modifying Protein-2 (RAMP2). J Biol Chem. 2015 Sep 18;290(38):23009-22. doi: 10.1074/jbc.M114.624601. Epub, 2015 Jul 21. PMID:26198634 doi:http://dx.doi.org/10.1074/jbc.M114.624601
- ↑ Zhang X, Stevens RC, Xu F. The importance of ligands for G protein-coupled receptor stability. Trends Biochem Sci. 2015 Feb;40(2):79-87. doi: 10.1016/j.tibs.2014.12.005. Epub, 2015 Jan 15. PMID:25601764 doi:http://dx.doi.org/10.1016/j.tibs.2014.12.005
- ↑ 'Lehninger A., Nelson D.N, & Cox M.M. (2008) Lehninger Principles of Biochemistry. W. H. Freeman, fifth edition.'
- ↑ 12.0 12.1 Salon JA, Lodowski DT, Palczewski K. The significance of G protein-coupled receptor crystallography for drug discovery. Pharmacol Rev. 2011 Dec;63(4):901-37. doi: 10.1124/pr.110.003350. PMID:21969326 doi:http://dx.doi.org/10.1124/pr.110.003350