We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox HEC
From Proteopedia
(Difference between revisions)
| Line 10: | Line 10: | ||
== Structure and Mechanism == | == Structure and Mechanism == | ||
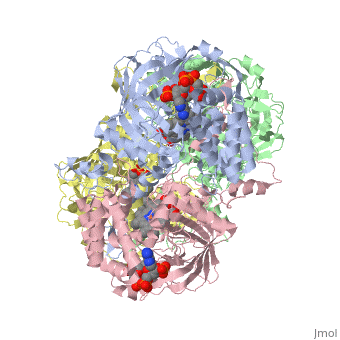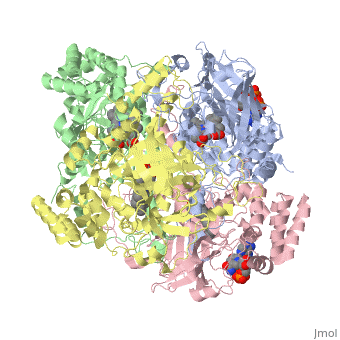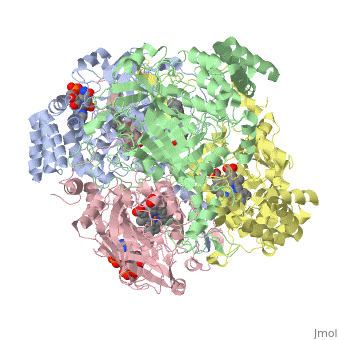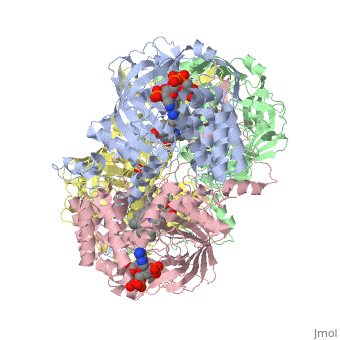
| - | Human erythrocyte catalase is a negatively charged heme-containing monofunctional tetrameric enzyme prevalent among aerobic organisms <ref name= Kodydková >PMID:25152049</ref><ref name=Alfonso-Prietro>PMID:22516655</ref><ref name=Dash>PMID:22521743</ref><ref name=Diaz>PMID:22209752 </ref><ref name=Nishikawa>PMID:19385054 </ref>([[Kodydková, Vávrová, Kocík, & Zák, A., 2014; Alfonso-Prietro, Vidossich, & Rovira, 2012; Dash & Phillips, 2012; Diaz, Loewen, Fita, & Carpena, 2012; Nishikawa, Hashida, & Takakura, 2009)]]. Stable forms of hydrogen peroxide are beneficial in biological reactions including hypoxia signal transduction, cell proliferation and differentiation regulation, and immune response mediation; however, it is toxic at high levels as free hydroxyl ions cannot be catalyzed by the body <ref name= Lennicke >PMID:26369938</ref><ref name= "halliwell">DOI: 10.1016/S0014-5739(00)02197</ref> ([[Lennicke et al., 2015]]; Halliwell, Clement, & Long, 2000). Within this catalytic group, hydrogen peroxide acts to both oxidize and reduce the reaction. Catalase ultimately functions to break down hydrogen peroxide<ref name="Dash" />(Dash & Phillips, 2012). This is accomplished in a two-step mechanism where the heme is first oxidized by a molecule of hydrogen peroxide to produce Compound I, a high energy oxyferryl cation radical intermediate, as well as a water molecule. Compound I is then immediately reduced by a second hydrogen peroxide molecule to produce a second molecule of water <ref name="Alfonso-Prietro" /><ref name="Diaz" /> (Alfonso-Prietro, Vidossich, & Rovira, 2012; Diaz, Loewen, Fita, & Carpena, 2012). The overall reaction results in two single-electron removal transfers from the iron atom of the heme group and the porphyrin from the oxoferryl radical, and a proton transfer from histidine. The mechanism is enthalpically driven by the distal histidine proton transfer as it is more exothermic than the electron transfers <ref name="Alfonso-Prietro" /><ref name="Diaz" /> (Alfonso-Prietro, Vidossich, & Rovira, 2012; Diaz, Loewen, Fita, & Carpena, 2012). | + | Human erythrocyte catalase is a negatively charged heme-containing monofunctional tetrameric enzyme prevalent among aerobic organisms <ref name= "Kodydková" >PMID:25152049</ref><ref name=Alfonso-Prietro>PMID:22516655</ref><ref name=Dash>PMID:22521743</ref><ref name=Diaz>PMID:22209752 </ref><ref name=Nishikawa>PMID:19385054 </ref>([[Kodydková, Vávrová, Kocík, & Zák, A., 2014; Alfonso-Prietro, Vidossich, & Rovira, 2012; Dash & Phillips, 2012; Diaz, Loewen, Fita, & Carpena, 2012; Nishikawa, Hashida, & Takakura, 2009)]]. Stable forms of hydrogen peroxide are beneficial in biological reactions including hypoxia signal transduction, cell proliferation and differentiation regulation, and immune response mediation; however, it is toxic at high levels as free hydroxyl ions cannot be catalyzed by the body <ref name= Lennicke >PMID:26369938</ref><ref name= "halliwell">DOI: 10.1016/S0014-5739(00)02197</ref> ([[Lennicke et al., 2015]]; Halliwell, Clement, & Long, 2000). Within this catalytic group, hydrogen peroxide acts to both oxidize and reduce the reaction. Catalase ultimately functions to break down hydrogen peroxide<ref name="Dash" />(Dash & Phillips, 2012). This is accomplished in a two-step mechanism where the heme is first oxidized by a molecule of hydrogen peroxide to produce Compound I, a high energy oxyferryl cation radical intermediate, as well as a water molecule. Compound I is then immediately reduced by a second hydrogen peroxide molecule to produce a second molecule of water <ref name="Alfonso-Prietro" /><ref name="Diaz" /> (Alfonso-Prietro, Vidossich, & Rovira, 2012; Diaz, Loewen, Fita, & Carpena, 2012). The overall reaction results in two single-electron removal transfers from the iron atom of the heme group and the porphyrin from the oxoferryl radical, and a proton transfer from histidine. The mechanism is enthalpically driven by the distal histidine proton transfer as it is more exothermic than the electron transfers <ref name="Alfonso-Prietro" /><ref name="Diaz" /> (Alfonso-Prietro, Vidossich, & Rovira, 2012; Diaz, Loewen, Fita, & Carpena, 2012). |
The catalase fold, a stereoscopic alignment of the clade 3 subunits, contains an eight-sheeted anti-parallel beta-barrel domain linked to a six alpha-helical domain via a lengthy protein sequence. Residues within β1-β4 contribute to the heme variant, while monomers within β5-β8 establish the NADPH binding site <ref name="Diaz" />(Diaz, Loewen, Fita, & Carpena, 2012). The positioning of the heme is determined by the proximal aromatic pyrrole compounds <scene name='3cs9/Overall_structure/1'>TextToBeDisplayed</scene>; in human erythrocyte catalase, catalytic His75 is positioned above pyrrole ring III, further producing a His-III orientation and heme-b variant. The NADPH binding site is located at the β,α-domain junction <ref name="Alfonso-Prietro" /><ref name="Diaz" /> (Alfonso-Prietro, Vidossich, & Rovira, 2012; Diaz, Loewen, Fita, & Carpena, 2012). When the NADPH molecule is bound, a right-handed clockwise helical formation is produced. In human erythrocyte catalase, only two of the four subunits allow for NADPH binding <ref name="Kodydková" /><ref name="Diaz" /> (Kodydková, Vávrová, Kocík, & Zák, A., 2014; Diaz, Loewen, Fita, & Carpena, 2012). The active site contains a negatively charged tyrosine and a positively charged histidine situated, respectively, proximal and distal to the heme group. The histidine is responsible for the formation of Compound I during the first step of the catalase mechanism <ref name="Alfonso-Prietro" /> (Alfonso-Prietro, Vidossich, & Rovira, 2012). | The catalase fold, a stereoscopic alignment of the clade 3 subunits, contains an eight-sheeted anti-parallel beta-barrel domain linked to a six alpha-helical domain via a lengthy protein sequence. Residues within β1-β4 contribute to the heme variant, while monomers within β5-β8 establish the NADPH binding site <ref name="Diaz" />(Diaz, Loewen, Fita, & Carpena, 2012). The positioning of the heme is determined by the proximal aromatic pyrrole compounds <scene name='3cs9/Overall_structure/1'>TextToBeDisplayed</scene>; in human erythrocyte catalase, catalytic His75 is positioned above pyrrole ring III, further producing a His-III orientation and heme-b variant. The NADPH binding site is located at the β,α-domain junction <ref name="Alfonso-Prietro" /><ref name="Diaz" /> (Alfonso-Prietro, Vidossich, & Rovira, 2012; Diaz, Loewen, Fita, & Carpena, 2012). When the NADPH molecule is bound, a right-handed clockwise helical formation is produced. In human erythrocyte catalase, only two of the four subunits allow for NADPH binding <ref name="Kodydková" /><ref name="Diaz" /> (Kodydková, Vávrová, Kocík, & Zák, A., 2014; Diaz, Loewen, Fita, & Carpena, 2012). The active site contains a negatively charged tyrosine and a positively charged histidine situated, respectively, proximal and distal to the heme group. The histidine is responsible for the formation of Compound I during the first step of the catalase mechanism <ref name="Alfonso-Prietro" /> (Alfonso-Prietro, Vidossich, & Rovira, 2012). | ||
Revision as of 01:48, 8 April 2016
1dgb
| |||||||||||