We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 427
From Proteopedia
(Difference between revisions)
| Line 23: | Line 23: | ||
The tertiary structure consists of mainly <scene name="48/483884/Alpha_helices/2">alpha helices</scene>, which can be seen in pink. The quaternary structure of the protein consists of <scene name='48/483884/Twosubunits/1'>two subunits</scene> forming a complex. The structure is about 58 kDA in size and made up of 458 amino acids. | The tertiary structure consists of mainly <scene name="48/483884/Alpha_helices/2">alpha helices</scene>, which can be seen in pink. The quaternary structure of the protein consists of <scene name='48/483884/Twosubunits/1'>two subunits</scene> forming a complex. The structure is about 58 kDA in size and made up of 458 amino acids. | ||
=====Alpha Helical Domains===== | =====Alpha Helical Domains===== | ||
| - | The Vitamin D binding protein consists of three alpha helical domains which are homologous. Domain I containing 10 aloha helices, Domain II 9, and Domain III 4 being shorter than the other domains. | + | The Vitamin D binding protein consists of three alpha helical domains which are homologous. Domain I containing 10 aloha helices, <scene name='48/483884/Domain_ii/3'>Domain II</scene> 9, and Domain III 4 being shorter than the other domains. |
=====Vitamin D Binding Protein and Human Serum Albumin===== | =====Vitamin D Binding Protein and Human Serum Albumin===== | ||
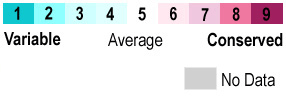
The overall structure is closely related to that of the human serum albumin, to which it is homologous. The proteins are very similar yet the three dimensional structure differs somewhat to facilitate binding. Looking at <scene name='48/483884/Evolutionaryconserved/2'>how the structure has evolved</scene> it can be seen that the outer edges are more variable while the core has more conserved sections. | The overall structure is closely related to that of the human serum albumin, to which it is homologous. The proteins are very similar yet the three dimensional structure differs somewhat to facilitate binding. Looking at <scene name='48/483884/Evolutionaryconserved/2'>how the structure has evolved</scene> it can be seen that the outer edges are more variable while the core has more conserved sections. | ||
Revision as of 01:43, 10 April 2016
| This Sandbox is Reserved from January 19, 2016, through August 31, 2016 for use for Proteopedia Team Projects by the class Chemistry 423 Biochemistry for Chemists taught by Lynmarie K Thompson at University of Massachusetts Amherst, USA. This reservation includes Sandbox Reserved 425 through Sandbox Reserved 439. |
Vitamin D binding protein (1j7e)[1]
Alex Debreceni, Robert Green, Uday Prakhya, Nicholas Rivelli, Elizabeth Swanson
Student Projects for UMass Chemistry 423 Spring 2016
| |||||||||||