We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox HEC
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
== Function == | == Function == | ||
Human erythrocyte catalase is used to protect hemoglobin by removing hydrogen peroxide generated from erythrocytes. Human catalase is a heme-containing enzyme whose primary function is to break down hydrogen peroxide into two molecules of water and one molecule of oxygen. Human catalase plays a major part in the defense against oxidative damage and inactivation of hemoglobin by removing half of the hydrogen peroxide formed by human erythrocytes <ref name="putnam">PMID:10656833</ref> . Hydrogen peroxide is a byproduct of normal cellular respiration, but is toxic at high concentrations. If catalase does not break down hydrogen peroxide, it gets converted into reactive oxygen species and can damage DNA, proteins, and cell membranes <ref name="goth">PMID:15771551</ref>. Human catalase enzyme has been noted as an important factor in inflammation, mutagenesis, prevention of apoptosis, and stimulation of tumors. During a normal catalytic cycle hydrogen peroxide is the source of both oxidative and reductive potential. NADPH has been known to also bind to human catalase, however it does not serve as the oxidative or reductive potential <ref name="putnam" /> [[(putnam)]]. | Human erythrocyte catalase is used to protect hemoglobin by removing hydrogen peroxide generated from erythrocytes. Human catalase is a heme-containing enzyme whose primary function is to break down hydrogen peroxide into two molecules of water and one molecule of oxygen. Human catalase plays a major part in the defense against oxidative damage and inactivation of hemoglobin by removing half of the hydrogen peroxide formed by human erythrocytes <ref name="putnam">PMID:10656833</ref> . Hydrogen peroxide is a byproduct of normal cellular respiration, but is toxic at high concentrations. If catalase does not break down hydrogen peroxide, it gets converted into reactive oxygen species and can damage DNA, proteins, and cell membranes <ref name="goth">PMID:15771551</ref>. Human catalase enzyme has been noted as an important factor in inflammation, mutagenesis, prevention of apoptosis, and stimulation of tumors. During a normal catalytic cycle hydrogen peroxide is the source of both oxidative and reductive potential. NADPH has been known to also bind to human catalase, however it does not serve as the oxidative or reductive potential <ref name="putnam" /> [[(putnam)]]. | ||
| + | |||
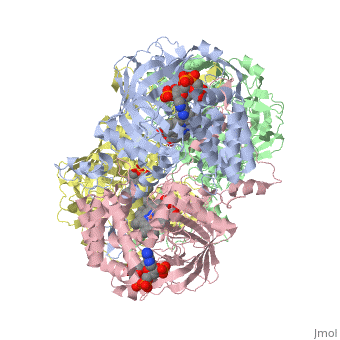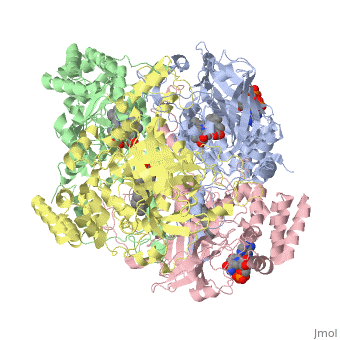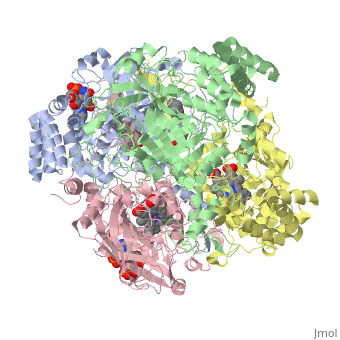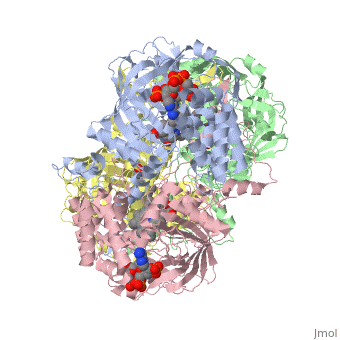
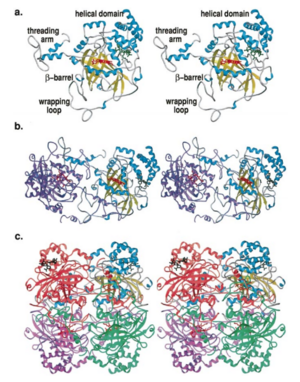
| + | [[Image:Hec.jpg.png | thumb |left|300px|'''Structure of Human Erythrocyte Catalase''' This figure represents a individual subunit of human catalase (a) , an arm-exchanged dimer with a catalase fold where both heme active sites are exposed on one surface (b), and a catalase tetramer with the addition of a second arm exchanged | ||
| + | dimer where the heme active site is buried within the enzyme. In this figure the beta-barrel domain is colored yellow, the alpha helices are blue, NADPH is dark green, and the active site heme is red. ]] | ||
| + | |||
== Structure == | == Structure == | ||
| - | + | ||
| + | |||
Human erythrocyte catalase is a negatively charged heme-containing monofunctional tetrameric enzyme that is prevalent among aerobic organisms <ref name= "Kodydková" >PMID:25152049</ref><ref name=Alfonso-Prietro>PMID:22516655</ref><ref name=Dash>PMID:22521743</ref><ref name=Diaz>PMID:22209752 </ref><ref name=Nishikawa>PMID:19385054 </ref>([[Kodydková, Vávrová, Kocík, & Zák, A., 2014; Alfonso-Prietro, Vidossich, & Rovira, 2012; Dash & Phillips, 2012; Diaz, Loewen, Fita, & Carpena, 2012; Nishikawa, Hashida, & Takakura, 2009)]]. The catalase fold, a stereoscopic alignment of the clade 3 subunits, contains an eight-sheeted anti-parallel beta-barrel domain linked to a six alpha-helical domain via a lengthy protein sequence. Residues within β1-β4 contribute to the heme variant, while monomers within β5-β8 establish the NADPH binding site <ref name="Diaz" />(Diaz, Loewen, Fita, & Carpena, 2012). The positioning of the heme is determined by the proximal aromatic pyrrole compounds; in human erythrocyte catalase, catalytic His75 is positioned above pyrrole ring III <scene name='3cs9/Overall_structure/1'>TextToBeDisplayed</scene>, further producing a His-III orientation and heme-b variant. The NADPH binding site is located at the β,α-domain junction <ref name="Alfonso-Prietro" /><ref name="Diaz" /> (Alfonso-Prietro, Vidossich, & Rovira, 2012; Diaz, Loewen, Fita, & Carpena, 2012). When the NADPH molecule is bound, a right-handed clockwise helical formation is produced. In human erythrocyte catalase, only two of the four subunits allow for NADPH binding <ref name="Kodydková" /><ref name="Diaz" /> (Kodydková, Vávrová, Kocík, & Zák, A., 2014; Diaz, Loewen, Fita, & Carpena, 2012). The active site contains a negatively charged tyrosine and a positively charged histidine situated, respectively, proximal and distal to the heme group. The histidine is responsible for the formation of Compound I during the first step of the catalase mechanism <ref name="Alfonso-Prietro" /> (Alfonso-Prietro, Vidossich, & Rovira, 2012). | Human erythrocyte catalase is a negatively charged heme-containing monofunctional tetrameric enzyme that is prevalent among aerobic organisms <ref name= "Kodydková" >PMID:25152049</ref><ref name=Alfonso-Prietro>PMID:22516655</ref><ref name=Dash>PMID:22521743</ref><ref name=Diaz>PMID:22209752 </ref><ref name=Nishikawa>PMID:19385054 </ref>([[Kodydková, Vávrová, Kocík, & Zák, A., 2014; Alfonso-Prietro, Vidossich, & Rovira, 2012; Dash & Phillips, 2012; Diaz, Loewen, Fita, & Carpena, 2012; Nishikawa, Hashida, & Takakura, 2009)]]. The catalase fold, a stereoscopic alignment of the clade 3 subunits, contains an eight-sheeted anti-parallel beta-barrel domain linked to a six alpha-helical domain via a lengthy protein sequence. Residues within β1-β4 contribute to the heme variant, while monomers within β5-β8 establish the NADPH binding site <ref name="Diaz" />(Diaz, Loewen, Fita, & Carpena, 2012). The positioning of the heme is determined by the proximal aromatic pyrrole compounds; in human erythrocyte catalase, catalytic His75 is positioned above pyrrole ring III <scene name='3cs9/Overall_structure/1'>TextToBeDisplayed</scene>, further producing a His-III orientation and heme-b variant. The NADPH binding site is located at the β,α-domain junction <ref name="Alfonso-Prietro" /><ref name="Diaz" /> (Alfonso-Prietro, Vidossich, & Rovira, 2012; Diaz, Loewen, Fita, & Carpena, 2012). When the NADPH molecule is bound, a right-handed clockwise helical formation is produced. In human erythrocyte catalase, only two of the four subunits allow for NADPH binding <ref name="Kodydková" /><ref name="Diaz" /> (Kodydková, Vávrová, Kocík, & Zák, A., 2014; Diaz, Loewen, Fita, & Carpena, 2012). The active site contains a negatively charged tyrosine and a positively charged histidine situated, respectively, proximal and distal to the heme group. The histidine is responsible for the formation of Compound I during the first step of the catalase mechanism <ref name="Alfonso-Prietro" /> (Alfonso-Prietro, Vidossich, & Rovira, 2012). | ||
Revision as of 22:31, 10 April 2016
1dgb
| |||||||||||