Sandbox HEC
From Proteopedia
(Difference between revisions)
| Line 20: | Line 20: | ||
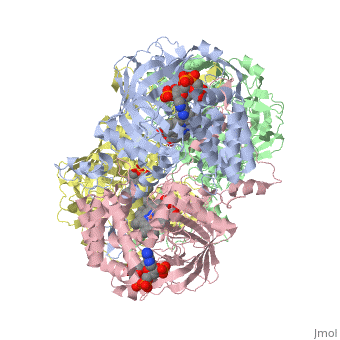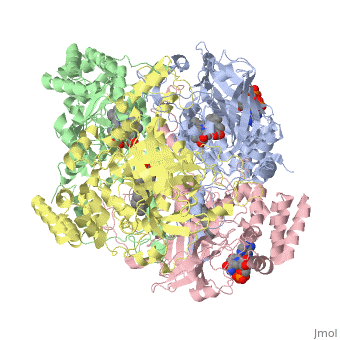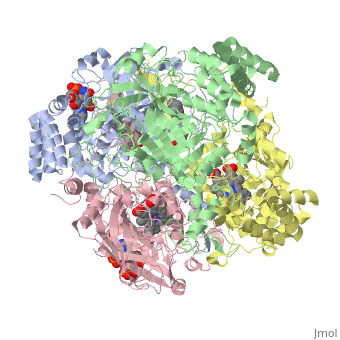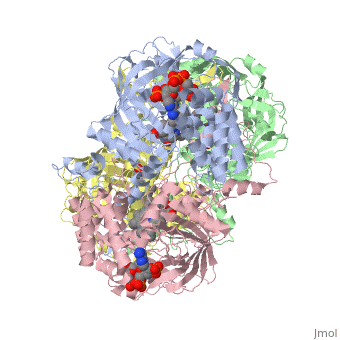
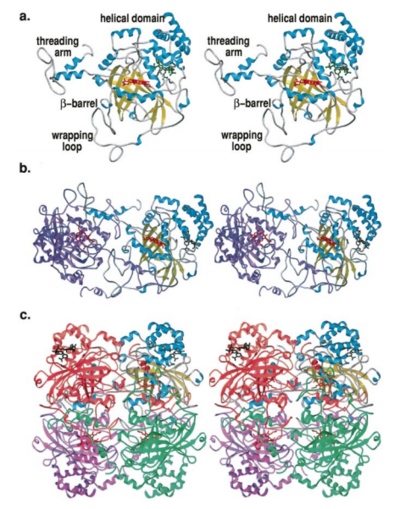
Human erythrocyte catalase is a negatively charged heme-containing monofunctional tetrameric enzyme that is prevalent among aerobic organisms <ref name= "Kodydková" >PMID:25152049</ref><ref name=Alfonso-Prietro>PMID:22516655</ref><ref name=Dash>PMID:22521743</ref><ref name=Diaz>PMID:22209752 </ref><ref name=Nishikawa>PMID:19385054 </ref>([[Kodydková, Vávrová, Kocík, & Zák, A., 2014; Alfonso-Prietro, Vidossich, & Rovira, 2012; Dash & Phillips, 2012; Diaz, Loewen, Fita, & Carpena, 2012; Nishikawa, Hashida, & Takakura, 2009)]]. The catalase fold, a stereoscopic alignment of the clade 3 subunits, contains an eight-sheeted anti-parallel beta-barrel domain linked to a six alpha-helical domain via a lengthy protein sequence. Residues within β1-β4 contribute to the heme variant, while monomers within β5-β8 establish the NADPH binding site <ref name="Diaz" />(Diaz, Loewen, Fita, & Carpena, 2012). The positioning of the heme is determined by the proximal aromatic pyrrole compounds; in human erythrocyte catalase, catalytic His75 is positioned above pyrrole ring III <scene name='3cs9/Overall_structure/1'>TextToBeDisplayed</scene>, further producing a His-III orientation and heme-b variant. The NADPH binding site is located at the β,α-domain junction <ref name="Alfonso-Prietro" /><ref name="Diaz" /> (Alfonso-Prietro, Vidossich, & Rovira, 2012; Diaz, Loewen, Fita, & Carpena, 2012). When the NADPH molecule is bound, a right-handed clockwise helical formation is produced. In human erythrocyte catalase, only two of the four subunits allow for NADPH binding <ref name="Kodydková" /><ref name="Diaz" /> (Kodydková, Vávrová, Kocík, & Zák, A., 2014; Diaz, Loewen, Fita, & Carpena, 2012). The active site contains a negatively charged tyrosine and a positively charged histidine situated, respectively, proximal and distal to the heme group. The histidine is responsible for the formation of Compound I during the first step of the catalase mechanism <ref name="Alfonso-Prietro" /> (Alfonso-Prietro, Vidossich, & Rovira, 2012). | Human erythrocyte catalase is a negatively charged heme-containing monofunctional tetrameric enzyme that is prevalent among aerobic organisms <ref name= "Kodydková" >PMID:25152049</ref><ref name=Alfonso-Prietro>PMID:22516655</ref><ref name=Dash>PMID:22521743</ref><ref name=Diaz>PMID:22209752 </ref><ref name=Nishikawa>PMID:19385054 </ref>([[Kodydková, Vávrová, Kocík, & Zák, A., 2014; Alfonso-Prietro, Vidossich, & Rovira, 2012; Dash & Phillips, 2012; Diaz, Loewen, Fita, & Carpena, 2012; Nishikawa, Hashida, & Takakura, 2009)]]. The catalase fold, a stereoscopic alignment of the clade 3 subunits, contains an eight-sheeted anti-parallel beta-barrel domain linked to a six alpha-helical domain via a lengthy protein sequence. Residues within β1-β4 contribute to the heme variant, while monomers within β5-β8 establish the NADPH binding site <ref name="Diaz" />(Diaz, Loewen, Fita, & Carpena, 2012). The positioning of the heme is determined by the proximal aromatic pyrrole compounds; in human erythrocyte catalase, catalytic His75 is positioned above pyrrole ring III <scene name='3cs9/Overall_structure/1'>TextToBeDisplayed</scene>, further producing a His-III orientation and heme-b variant. The NADPH binding site is located at the β,α-domain junction <ref name="Alfonso-Prietro" /><ref name="Diaz" /> (Alfonso-Prietro, Vidossich, & Rovira, 2012; Diaz, Loewen, Fita, & Carpena, 2012). When the NADPH molecule is bound, a right-handed clockwise helical formation is produced. In human erythrocyte catalase, only two of the four subunits allow for NADPH binding <ref name="Kodydková" /><ref name="Diaz" /> (Kodydková, Vávrová, Kocík, & Zák, A., 2014; Diaz, Loewen, Fita, & Carpena, 2012). The active site contains a negatively charged tyrosine and a positively charged histidine situated, respectively, proximal and distal to the heme group. The histidine is responsible for the formation of Compound I during the first step of the catalase mechanism <ref name="Alfonso-Prietro" /> (Alfonso-Prietro, Vidossich, & Rovira, 2012). | ||
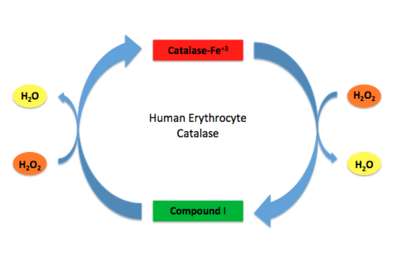
| + | [[Image:HEC mechanism.jpg.png|thumb|400px|'''Human Erythrocyte Catalase Mechanism''' This figure illustrates the two step mechanism of catalase. ]] | ||
== Mechanism == | == Mechanism == | ||
| + | |||
| + | |||
| + | |||
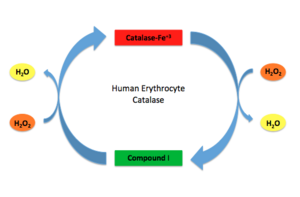
Stable forms of hydrogen peroxide are beneficial in biological reactions including hypoxia signal transduction, cell proliferation and differentiation regulation, as well as immune response mediation; however, it is toxic at high levels as free hydroxyl ions cannot be catalyzed by the body <ref name= Lennicke >PMID:26369938</ref><ref name= "halliwell">DOI: 10.1016/S0014-5739(00)02197</ref> ([[Lennicke et al., 2015]]; Halliwell, Clement, & Long, 2000). Within this catalytic group, hydrogen peroxide acts to both oxidize and reduce the reaction. Catalase ultimately functions to break down hydrogen peroxide<ref name="Dash" />(Dash & Phillips, 2012). This is accomplished in a two-step mechanism where the heme is first oxidized by a molecule of hydrogen peroxide to produce Compound I, a high energy oxyferryl cation radical intermediate, as well as a water molecule. Compound I is then immediately reduced by a second hydrogen peroxide molecule to produce a second molecule of water <ref name="Alfonso-Prietro" /><ref name="Diaz" /> (Alfonso-Prietro, Vidossich, & Rovira, 2012; Diaz, Loewen, Fita, & Carpena, 2012). The overall reaction results in two single-electron removal transfers from the iron atom of the heme group and the porphyrin from the oxoferryl radical, as well as a proton transfer from histidine. The mechanism is enthalpically driven by the distal histidine proton transfer as it is more exothermic than the electron transfers <ref name="Alfonso-Prietro" /><ref name="Diaz" /> (Alfonso-Prietro, Vidossich, & Rovira, 2012; Diaz, Loewen, Fita, & Carpena, 2012). | Stable forms of hydrogen peroxide are beneficial in biological reactions including hypoxia signal transduction, cell proliferation and differentiation regulation, as well as immune response mediation; however, it is toxic at high levels as free hydroxyl ions cannot be catalyzed by the body <ref name= Lennicke >PMID:26369938</ref><ref name= "halliwell">DOI: 10.1016/S0014-5739(00)02197</ref> ([[Lennicke et al., 2015]]; Halliwell, Clement, & Long, 2000). Within this catalytic group, hydrogen peroxide acts to both oxidize and reduce the reaction. Catalase ultimately functions to break down hydrogen peroxide<ref name="Dash" />(Dash & Phillips, 2012). This is accomplished in a two-step mechanism where the heme is first oxidized by a molecule of hydrogen peroxide to produce Compound I, a high energy oxyferryl cation radical intermediate, as well as a water molecule. Compound I is then immediately reduced by a second hydrogen peroxide molecule to produce a second molecule of water <ref name="Alfonso-Prietro" /><ref name="Diaz" /> (Alfonso-Prietro, Vidossich, & Rovira, 2012; Diaz, Loewen, Fita, & Carpena, 2012). The overall reaction results in two single-electron removal transfers from the iron atom of the heme group and the porphyrin from the oxoferryl radical, as well as a proton transfer from histidine. The mechanism is enthalpically driven by the distal histidine proton transfer as it is more exothermic than the electron transfers <ref name="Alfonso-Prietro" /><ref name="Diaz" /> (Alfonso-Prietro, Vidossich, & Rovira, 2012; Diaz, Loewen, Fita, & Carpena, 2012). | ||
The deeply buried heme group is connected to the protein surface by a primary channel which provides a transport pathway for the hydrogen peroxide substrate <ref name="Diaz" />(Diaz, Loewen, Fita, & Carpena, 2012). The transportation of hydrogen peroxide through the main channel is regulated by electrical dipole interactions between the hydrogen peroxide and the hydrophobic portion of the channel containing negatively charged aspartate and positively charged iron from the heme <ref name="Lennicke" /><ref name="Diaz" /><ref name="halliwell" />(Lennicke et al., 2015; Diaz, Loewen, Fita, & Carpena, 2012; Halliwell, Clement, & Long, 2000). Additionally, less significant lateral channels allow products to leave the heme pocket<ref name="Diaz" /> (Diaz, Loewen, Fita, & Carpena, 2012). Human erythrocyte catalase is not evenly distributed throughout the body due to restricted endothelium passageways; this allows for a controlled and localized spread of the protein<ref name="Nishikawa" /> (Nishikawa, Hashida, & Takakura, 2009). | The deeply buried heme group is connected to the protein surface by a primary channel which provides a transport pathway for the hydrogen peroxide substrate <ref name="Diaz" />(Diaz, Loewen, Fita, & Carpena, 2012). The transportation of hydrogen peroxide through the main channel is regulated by electrical dipole interactions between the hydrogen peroxide and the hydrophobic portion of the channel containing negatively charged aspartate and positively charged iron from the heme <ref name="Lennicke" /><ref name="Diaz" /><ref name="halliwell" />(Lennicke et al., 2015; Diaz, Loewen, Fita, & Carpena, 2012; Halliwell, Clement, & Long, 2000). Additionally, less significant lateral channels allow products to leave the heme pocket<ref name="Diaz" /> (Diaz, Loewen, Fita, & Carpena, 2012). Human erythrocyte catalase is not evenly distributed throughout the body due to restricted endothelium passageways; this allows for a controlled and localized spread of the protein<ref name="Nishikawa" /> (Nishikawa, Hashida, & Takakura, 2009). | ||
| - | + | [[Image:HEC mechanism.jpg.png|thumb|300px|'''Human Erythrocyte Catalase Mechanism''' This figure illustrates the two step mechanism of catalase. ]] | |
== Disease and Disorders == | == Disease and Disorders == | ||
There are 12 known mutations in the human erythrocyte catalase gene that have been found to cause acatalasemia <ref name="László Góth">PMID:22365890</ref> (5). Acatalasemia is an autosomal recessive condition in which human erythrocyte catalase levels are very low and occurs in individuals that are homozygous at the catalase gene locus. Most people are asymptomatic and are diagnosed because a family member is affected. However, although they are asymptomatic, they have an increased risk of chronic diseases. | There are 12 known mutations in the human erythrocyte catalase gene that have been found to cause acatalasemia <ref name="László Góth">PMID:22365890</ref> (5). Acatalasemia is an autosomal recessive condition in which human erythrocyte catalase levels are very low and occurs in individuals that are homozygous at the catalase gene locus. Most people are asymptomatic and are diagnosed because a family member is affected. However, although they are asymptomatic, they have an increased risk of chronic diseases. | ||
Revision as of 01:30, 11 April 2016
1dgb
| |||||||||||