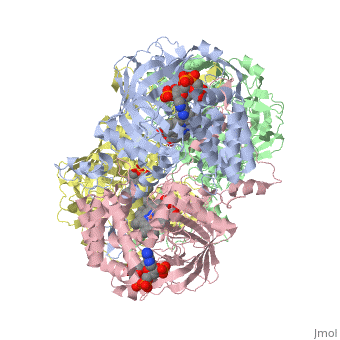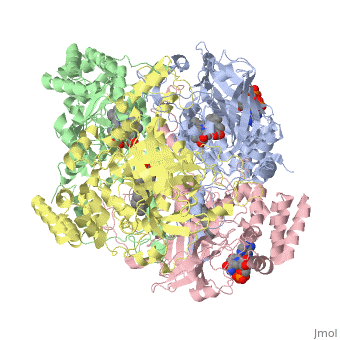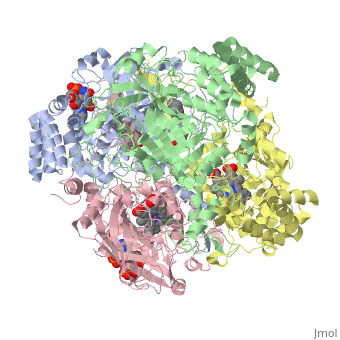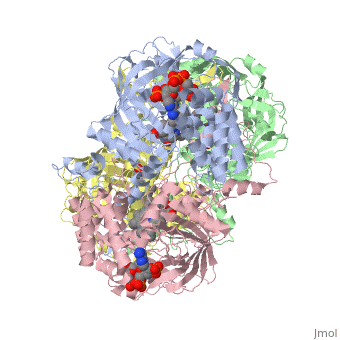
Human Erythrocyte Catalase
Human Erythrocyte Catalase is an enzyme whose main function is to break down hydrogen peroxide that is produced as a byproduct of metabolism[1]. Catalase can break down approximately one million molecules of hydrogen peroxide per second [2]. Without catalase hydrogen peroxide would build up and cause damage to the cell and could even signal it to initiate cell death [1]. Structurally it contains four subunits each with a heme group in the center which allows it to break down the peroxide in human cells [2].
Function
Human erythrocyte catalase is used to protect hemoglobin by removing hydrogen peroxide generated from erythrocytes. Human catalase is a heme-containing enzyme whose primary function is to break down hydrogen peroxide into two molecules of water and one molecule of oxygen. Human catalase plays a major part in the defense against oxidative damage and inactivation of hemoglobin by removing half of the hydrogen peroxide formed by human erythrocytes [3] . Hydrogen peroxide is a byproduct of normal cellular respiration, but is toxic at high concentrations. If catalase does not break down hydrogen peroxide, it gets converted into reactive oxygen species and can damage DNA, proteins, and cell membranes [4]. Human catalase enzyme has been noted as an important factor in inflammation, mutagenesis, prevention of apoptosis, and stimulation of tumors. During a normal catalytic cycle hydrogen peroxide is the source of both oxidative and reductive potential. NADPH has been known to also bind to human catalase, however, it does not serve as the oxidative or reductive potential, but protects the catalase from being inactivated by hydrogen peroxide [3] (putnam).
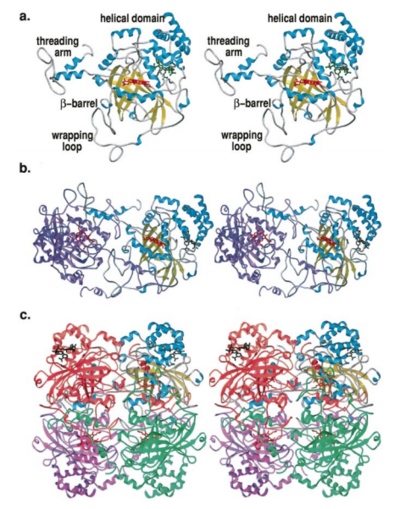
Structure of Human Erythrocyte Catalase This figure represents a individual subunit of human catalase (a) , an arm-exchanged dimer with a catalase fold where both heme active sites are exposed on one surface (b), and a catalase tetramer with the addition of a second arm exchanged dimer where the heme active site is buried within the enzyme. In this figure the beta-barrel domain is colored yellow, the alpha helices are blue, NADPH is dark green, and the active site heme is red.
[3]
Structure
Human erythrocyte catalase is a negatively charged heme-containing monofunctional tetrameric enzyme that is prevalent among aerobic organisms [5][2][6][7][8](Kodydková, Vávrová, Kocík, & Zák, A., 2014; Alfonso-Prietro, Vidossich, & Rovira, 2012; Dash & Phillips, 2012; Diaz, Loewen, Fita, & Carpena, 2012; Nishikawa, Hashida, & Takakura, 2009). The catalase fold, a stereoscopic alignment of the clade 3 subunits, contains an eight-sheeted anti-parallel beta-barrel domain linked to a six alpha-helical domain via a lengthy protein sequence. Residues within β1-β4 contribute to the heme variant, while monomers within β5-β8 establish the NADPH binding site [7](Diaz, Loewen, Fita, & Carpena, 2012). The positioning of the heme is determined by the proximal aromatic pyrrole compounds; in human erythrocyte catalase, catalytic His75 is positioned above pyrrole ring III , further producing a His-III orientation and heme-b variant. The NADPH binding site is located at the β,α-domain junction [2][7] (Alfonso-Prietro, Vidossich, & Rovira, 2012; Diaz, Loewen, Fita, & Carpena, 2012). When the NADPH molecule is bound, a right-handed clockwise helical formation is produced. In human erythrocyte catalase, only two of the four subunits allow for NADPH binding [5][7] (Kodydková, Vávrová, Kocík, & Zák, A., 2014; Diaz, Loewen, Fita, & Carpena, 2012). The active site contains a negatively charged tyrosine and a positively charged histidine situated, respectively, proximal and distal to the heme group. The histidine is responsible for the formation of Compound I during the first step of the catalase mechanism [2] (Alfonso-Prietro, Vidossich, & Rovira, 2012).
Mechanism
Stable forms of hydrogen peroxide are beneficial in biological reactions including hypoxia signal transduction, cell proliferation and differentiation regulation, as well as immune response mediation; however, it is toxic at high levels as free hydroxyl ions cannot be catalyzed by the body [9][10] (Lennicke et al., 2015; Halliwell, Clement, & Long, 2000). Within this catalytic group, hydrogen peroxide acts to both oxidize and reduce the reaction. Catalase ultimately functions to break down hydrogen peroxide[6](Dash & Phillips, 2012). This is accomplished in a two-step mechanism where the heme is first oxidized by a molecule of hydrogen peroxide to produce Compound I, a high energy oxyferryl cation radical intermediate, as well as a water molecule. Compound I is then immediately reduced by a second hydrogen peroxide molecule to produce a second molecule of water [2][7] (Alfonso-Prietro, Vidossich, & Rovira, 2012; Diaz, Loewen, Fita, & Carpena, 2012). The overall reaction results in two single-electron removal transfers from the iron atom of the heme group and the porphyrin from the oxoferryl radical, as well as a proton transfer from histidine. The mechanism is enthalpically driven by the distal histidine proton transfer as it is more exothermic than the electron transfers [2][7] (Alfonso-Prietro, Vidossich, & Rovira, 2012; Diaz, Loewen, Fita, & Carpena, 2012).
The deeply buried heme group is connected to the protein surface by a primary channel which provides a transport pathway for the hydrogen peroxide substrate [7](Diaz, Loewen, Fita, & Carpena, 2012). The transportation of hydrogen peroxide through the main channel is regulated by electrical dipole interactions between the hydrogen peroxide and the hydrophobic portion of the channel containing negatively charged aspartate and positively charged iron from the heme [9][7][10](Lennicke et al., 2015; Diaz, Loewen, Fita, & Carpena, 2012; Halliwell, Clement, & Long, 2000). Additionally, less significant lateral channels allow products to leave the heme pocket[7] (Diaz, Loewen, Fita, & Carpena, 2012). Human erythrocyte catalase is not evenly distributed throughout the body due to restricted endothelium passageways; this allows for a controlled and localized spread of the protein[8] (Nishikawa, Hashida, & Takakura, 2009).
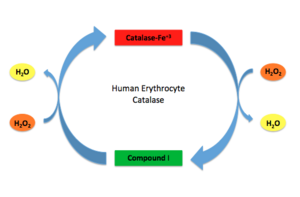
Human Erythrocyte Catalase Mechanism This figure illustrates the two step mechanism of catalase.
Disease and Disorders
There are 12 known mutations in the human erythrocyte catalase gene that have been found to cause acatalasemia [11] (5). Acatalasemia is an autosomal recessive condition in which human erythrocyte catalase levels are very low and occurs in individuals that are homozygous at the catalase gene locus. Most people are asymptomatic and are diagnosed because a family member is affected. However, although they are asymptomatic, they have an increased risk of chronic diseases.
Acatalasemia can be correlated with ulcers and gangrene. When this occurs, the condition is known as Takahara disease. Ulcers and gangrene can result from high levels of hydrogen peroxide that is normally produced from bacteria. Mutations in the human erythrocyte catalase gene tend to reduce the activity of human erythrocyte catalase [12] (1) to less than 10% of its normal activity, thus reducing the enzymes ability to degrade hydrogen peroxide and causing it to build-up. This build-up in turn causes ulcers and gangrene.
A similar condition to acatalasemia is hypocatalasemia, in which a individual is heterozygous at the mutated catalase gene instead of homozygous. This mutation cuts the activity of human erythrocyte catalase by half. Similar to acatalasemia, this condition normally doesn’t cause health issues [13](2).
Acatalasemia is also associated with type 2 diabetes mellitus, the most common form of diabetes. The build-up of hydrogen peroxide from the decrease in human erythrocyte catalase activity can damage beta cells in the pancreas. The pancreas releases insulin, which helps your body regulate your blood sugar level. However, the damaged beta cells cannot utilize the insulin as well as normal beta cells, which leads to type 2 diabetes mellitus. These defective beta cells are thought to be why people with acatalasemia have an increased risk for type 2 diabetes mellitus. A larger percentage of people with diabetes have acatalasemia than those without. Those with acatalasemia also tend to develop diabetes at an earlier age [12] (1).
Common variations in the human erythrocyte gene and variations in the regions of DNA that help to regulate the gene’s activity may also lead to an increased risk of a person developing specific common, complex diseases such as hypertension, osteoporosis, heart attack and stroke due to the elevated levels of cholesterol and other fats in the blood [12] (1,3,4). However, not all people experience health problems when they have a loss of catalase activity and others do not have an identified mutation in the human erythrocyte catalase gene when they have a loss in catalase activity. The cause of both of these situations is unclear. Some research hypothesized that the activity is also influenced by other genetic factors as well as environmental conditions[12] (1,5).
References
- ↑ 1.0 1.1 Yazdi F, Minai-Tehrani D, Jahngirvand M, Almasirad A, Mousavi Z, Masoud M, Mollasalehi H. Functional and structural changes of human erythrocyte catalase induced by cimetidine: proposed model of binding. Mol Cell Biochem. 2015 Jun;404(1-2):97-102. doi: 10.1007/s11010-015-2369-3. Epub , 2015 Mar 5. PMID:25739358 doi:http://dx.doi.org/10.1007/s11010-015-2369-3
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Alfonso-Prieto M, Vidossich P, Rovira C. The reaction mechanisms of heme catalases: an atomistic view by ab initio molecular dynamics. Arch Biochem Biophys. 2012 Sep 15;525(2):121-30. doi: 10.1016/j.abb.2012.04.004. , Epub 2012 Apr 10. PMID:22516655 doi:http://dx.doi.org/10.1016/j.abb.2012.04.004
- ↑ 3.0 3.1 3.2 Putnam CD, Arvai AS, Bourne Y, Tainer JA. Active and inhibited human catalase structures: ligand and NADPH binding and catalytic mechanism. J Mol Biol. 2000 Feb 11;296(1):295-309. PMID:10656833 doi:http://dx.doi.org/10.1006/jmbi.1999.3458
- ↑ Goth L, Rass P, Pay A. Catalase enzyme mutations and their association with diseases. Mol Diagn. 2004;8(3):141-9. PMID:15771551
- ↑ 5.0 5.1 Kodydkova J, Vavrova L, Kocik M, Zak A. Human catalase, its polymorphisms, regulation and changes of its activity in different diseases. Folia Biol (Praha). 2014;60(4):153-67. PMID:25152049
- ↑ 6.0 6.1 Dash B, Phillips TD. Molecular characterization of a catalase from Hydra vulgaris. Gene. 2012 Jun 15;501(2):144-52. doi: 10.1016/j.gene.2012.04.015. Epub 2012 Apr, 13. PMID:22521743 doi:http://dx.doi.org/10.1016/j.gene.2012.04.015
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 7.8 Diaz A, Loewen PC, Fita I, Carpena X. Thirty years of heme catalases structural biology. Arch Biochem Biophys. 2012 Sep 15;525(2):102-10. doi: 10.1016/j.abb.2011.12.011. , Epub 2011 Dec 23. PMID:22209752 doi:http://dx.doi.org/10.1016/j.abb.2011.12.011
- ↑ 8.0 8.1 Nishikawa M, Hashida M, Takakura Y. Catalase delivery for inhibiting ROS-mediated tissue injury and tumor metastasis. Adv Drug Deliv Rev. 2009 Apr 28;61(4):319-26. PMID:19385054
- ↑ 9.0 9.1 Lennicke C, Rahn J, Lichtenfels R, Wessjohann LA, Seliger B. Hydrogen peroxide - production, fate and role in redox signaling of tumor cells. Cell Commun Signal. 2015 Sep 14;13:39. doi: 10.1186/s12964-015-0118-6. PMID:26369938 doi:http://dx.doi.org/10.1186/s12964-015-0118-6
- ↑ 10.0 10.1 doi: https://dx.doi.org/10.1016/S0014-5739(00)02197
- ↑ Goth L, Nagy T. Acatalasemia and diabetes mellitus. Arch Biochem Biophys. 2012 Sep 15;525(2):195-200. doi: 10.1016/j.abb.2012.02.005., Epub 2012 Feb 16. PMID:22365890 doi:http://dx.doi.org/10.1016/j.abb.2012.02.005
- ↑ 12.0 12.1 12.2 12.3 Goth L, Nagy T. Inherited catalase deficiency: is it benign or a factor in various age related disorders? Mutat Res. 2013 Oct-Dec;753(2):147-54. doi: 10.1016/j.mrrev.2013.08.002. Epub, 2013 Sep 8. PMID:24025477 doi:http://dx.doi.org/10.1016/j.mrrev.2013.08.002
- ↑ Goth L, Eaton JW. Hereditary catalase deficiencies and increased risk of diabetes. Lancet. 2000 Nov 25;356(9244):1820-1. PMID:11117918 doi:http://dx.doi.org/10.1016/S0140-6736(00)03238-4