Introduction
The goal of pharmaceuticals is to prevent or cure disease through drug therapy by specifically targeting cells, proteins, enzymes, genes, etc. It is often crucial to understand the structure, function, and relevant mechanisms involved with the target when designing an effective drug candidate. Furthermore, being able to know the effects on structure after drug-binding can provide insight into the functionality of a specific target. Because of this fact, it is common practice for research labs to develop protein crystals and use methods like x-ray diffraction, electron density mapping, and nuclear magnetic resonance spectroscopy to model the full structure. In this case, P2Y12, a member of P2Y receptor, was modeled when binded to AZD1283, an engineered receptor inhibitor.The molecular scene shows the chemical of P2Y12 with the anionic side chains in red and charged nucleic acids and ligands in grey for contrast.
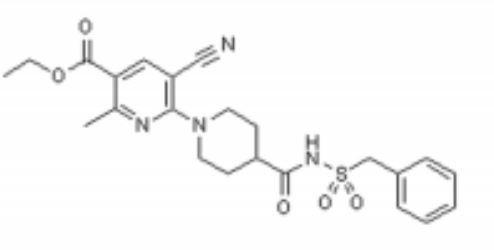
Image analysis of P2Y12 crystals is used to model protein structure in complex with AstraZeneca’s novel AZD1283: Ethyl 6-(4(-((benzylsulphonyl)carbamoyl)piperidin-1yl)-5-cyano-2-methylnicotinate. AZD1283 functions to block the P2Y12 receptor as a means to treat thrombosis. AZD1283-binding leads to unique protein structure, unfound in other P2Y receptors. Helix V of seven transmembrane helices is found to be . This change along with the discovery of a potential second active within P2Y12 has implications on how P2Y12 uses it’s seven transmembrane helical bundle interact with ADP in the bloodstream.
As a member of a large class of G-protein-coupled receptors, P2Y12 is often an initial role player in signal transduction and cellular response due to external environmental factors. In the case of P2Y12, the receptor responds to ADP concentrations in the extracellular matrix and on a larger scale the blood stream. There is importance is understanding how P2Y12's structure receives ADP as an activator. In turn, knowledge of how P2Y12 is affected when properly inhibited can lead to improved drug design in terms of bioavailability, binding affinity, and effectiveness of inhibition. Ultimately, pharmaceuticals will be better able to prevent and treat cardiovascular diseases and medical conditions (thrombosis, hypercoagulable states) and more immediate dangers (stroke, embolism, and heart attacks).
Overall Structure
P2Y12R is a 1 chain structure. The of P2Y12R consists of eight alpha helices. Seven transmembrane alpha helices are tilted and in a bundle, while the carboxy-terminal helix VIII is parallel to the membrane bilayer. The demonstrates how the chain goes from the N to C termini with each helix being approximately one color each of the color scheme.
P2Y12R contains only one disulfide bond that connects the amino terminus with helix VII shown . There are also two cholesterol molecules that are bound to two receptor molecules. One is bound between helix III and helix V and the other bound between helix I and helix VII.
P2Y12R has some distinctive features from other GPCR structures in its family. Helix V, for example, has around two more helical turns and does not have the typical helical bend that other GPCR structures have. As mentioned above, helix V is because the structure lacks proline and glycine residues to destabilize its structure. Furthermore, the elongated and straightened conformation causes P2Y12R’s extracellular end to shift 6 Å closer to helix IV compared to other class A GPCR structures. In addition, the intracellular tip of helix VII is closer to the seven transmembrane helical bundle. Helix VI’s intracellular tip is tilted slightly outward and shifted closer to the intracellular surface than other GPCR structures.
This demonstrates the polar and nonpolar regions of the P2Y12R's structure. AZD1283 spans more than 17 Å between helix IV and helix VII contributing to the polar and hydrophobic bonding with helices III–VI.
Binding Interactions
The interaction between P2Y12 and AZD1283 is different in P2Y12 binding pocket and its PAR1 equivalent. PAR1's 24 residues of ECL2 have more interaction in binding, while 16 unresolved residues ECL2 of P2Y12 is less likely to interact with AZD1283. Moreover, the shifted outward of helicies IV, VI and VII due to the extracellular make AZD1283 binds deeper into 7TM domain. It formed two pockets for the binding of AZD1283, separated by residues Y105 and K280, with pocket 1 consist of helices III-VII, while pocket 2 consists of helices I-III and VII. Among them, pocket 1 take part in the binding of AZD1283 and P2Y12, while pocket 2 does not.
The binding between P2Y12 and AZD1283 is also different from other GPCRs. The 17A elongated ligand is between helices IV and VII, which belongs in pocket 1. The antagonist AZD1283's piperidinyl-nicotinate group is inserted into sub-pocket of helices III, IV and V; while the benzylsulphonyl group interacts with helices VI and VII, forming at least seven polar and ionic interaction between P2Y12 and AZD1283.
and C175 (two cysteine residues in helix III and ECL2 of P2Y12, respectively) are also notable in P2Y12-AZD1283 complexes. The two cysteines are highly conserved in GPCR family, and they form a disulphide bond in all GPCRs. But for P2Y12, no electron density is observed at C97, which means the disulphide bond in P2Y12 would be different. Moreover, mutation at C97 and C175 does not change the protein yield and stability, while increasing the melting temperature when in complex in AZD1283, which indicates that the mutation in both cysteine does not alter P2Y12 binding ability with AZD1283.
Additional Features
Acute coronary syndrome, a condition in which there is sudden blockage of blood flow to the heart, is majorly caused by a disease known as atherothrombosis. This disease is characterized by blocked arteries due to thrombosis: formation of a clot within a blood vessel. The P2Y12 protein is an important platelet receptor inhibitor that can be combined with aspirin in the management of ACS. It regulates certain functions through purinergic signaling, which is a form of extracellular signaling mediated by purine nucleotides and nucleosides like ADP and ATP.[5]
The P2Y12 receptor is mainly found on the surface of blood platelets, and functions as a regulator in platelet activation and blood clotting. The P2Y12 receptor is a G-protein coupled receptor, (characterized by seven alpha helices each) linked to the cAMP-signaling pathway. It mediates platelet activation by decreasing intracellular cAMP levels through inhibition of an AC-mediated signaling pathway.[1] Acting as a chemoreceptor, P2Y12 utilizes ADP as an agonist, which initiates ADP-induced platelet aggregation. Clopidogrel in covalent binding complex with P2Y12 acts as an anti platelet agent, specifically as an ADP receptor inhibitor to decrease platelet aggregation and inhibit thrombus formation.[3]
Clopidogrel is a pro-drug and thienopyridine-type inhibitor of the P2Y12 receptor, which requires Cytochrome P450 to hepatically transform it to exert its anti platelet effect.[4] is a membrane protein, characteristic of alternating hydrophobic and polar groups. The central heme group is stabilized by several side chains. The Fe2+ atom that makes up the heart of the heme group is surrounded by a highly hydrophobic porphyrin ring. The role of the heme group in biological systems is to facilitate oxygen transport as well as aid in electron transfer as part of the electron transport chain. The heme group of the hemeprotein Cytochrome P450 acts as a catalyst for the metabolism of clopidogrel.[2] Shown below is the activation of Clopidogrel in vivo. CYP2C19 is an enzymatic member of the cytochrome P450 mixed-function oxidase system. The final step is a hydrolysis to yield the active metabolite.
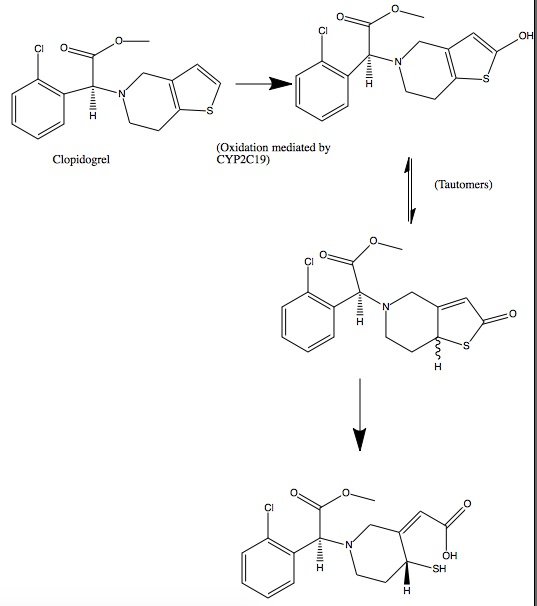
Quiz Question 1
Below is the structure for AZD1283. shows a representation of the pocket where AZD1283 binds to P2Y12R. The dark salmon molecule is AZD1283 which is surrounded by magenta colored amino acids which are polar and gray colored ones which are hydrophobic.List 3 amino acid residues from the scene that should participate in hydrogen bonding with AZD1283 and 3 that should not. Why might the amino acids that don't hydrogen bond with AZD1283 still be present in the pocket?
See Also
Credits
Introduction - Adam Murphy
Overall Structure - Cora Ricker
Drug Binding Site - Duy Nguyen
Additional Features - Lauren Timmins
Quiz Question 1 - Aidan Finnerty
References
- ↑ Zhang K, Zhang J, Gao ZG, Zhang D, Zhu L, Han GW, Moss SM, Paoletta S, Kiselev E, Lu W, Fenalti G, Zhang W, Muller CE, Yang H, Jiang H, Cherezov V, Katritch V, Jacobson KA, Stevens RC, Wu B, Zhao Q. Structure of the human P2Y12 receptor in complex with an antithrombotic drug. Nature. 2014 May 1;509(7498):115-8. doi: 10.1038/nature13083. Epub 2014 Mar 23. PMID:24670650 doi:http://dx.doi.org/10.1038/nature13083
1. Savi, P., et al. "P2Y 12, a new platelet ADP receptor, target of clopidogrel." Biochemical and biophysical research communications 283.2 (2001): 379-383.
2. Coon, Minor J. "Cytochrome P450: nature's most versatile biological catalyst." Annu. Rev. Pharmacol. Toxicol. 45 (2005): 1-25.
3. Gurbel, Paul A., et al. "Clopidogrel for coronary stenting response variability, drug resistance, and the effect of pretreatment platelet reactivity." Circulation 107.23 (2003): 2908-2913.
4. Barn, Kulpreet, and Steven R. Steinhubl. "A brief review of the past and future of platelet P2Y12 antagonist." Coronary artery disease 23.6 (2012): 368-374.
5. Bodin, Philippe, and Geoffrey Burnstock. "Purinergic signalling: ATP release." Neurochemical research 26.8-9 (2001): 959-969.


