User:Kurt Corsbie/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
<scene name='72/726409/Overview/5'>mGlu<sub>5</sub></scene> is seen as a [https://en.wikipedia.org/wiki/Protein_dimer homodimer] ''in vivo,'' with each subunit being comprised of three domains: extracellular, trans-membrane and cysteine-rich. mGlu<sub>5</sub> is centered on the trans-membrane domain, comprised of seven α-helices all roughly parallel.<ref name="Primary">PMID: 25042998 </ref> Also displayed is Intracellular Loop (ICL) 1 which forms a short α-helix and interacts directly with a trans-membrane helix to stabilize mGlu<sub>5</sub>’s conformation. Additionally, ICL3 and Extracellular Loops (ECL) 1 and 3 all lack secondary structure, and ECL2 interacts with trans-membrane (TM) helices 1, 2, and 3 as well as ECL 1,again helping to stabilize the protein’s overall conformation.<ref name="Primary">PMID: 25042998 </ref> | <scene name='72/726409/Overview/5'>mGlu<sub>5</sub></scene> is seen as a [https://en.wikipedia.org/wiki/Protein_dimer homodimer] ''in vivo,'' with each subunit being comprised of three domains: extracellular, trans-membrane and cysteine-rich. mGlu<sub>5</sub> is centered on the trans-membrane domain, comprised of seven α-helices all roughly parallel.<ref name="Primary">PMID: 25042998 </ref> Also displayed is Intracellular Loop (ICL) 1 which forms a short α-helix and interacts directly with a trans-membrane helix to stabilize mGlu<sub>5</sub>’s conformation. Additionally, ICL3 and Extracellular Loops (ECL) 1 and 3 all lack secondary structure, and ECL2 interacts with trans-membrane (TM) helices 1, 2, and 3 as well as ECL 1,again helping to stabilize the protein’s overall conformation.<ref name="Primary">PMID: 25042998 </ref> | ||
===Key Interactions=== | ===Key Interactions=== | ||
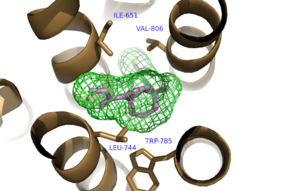
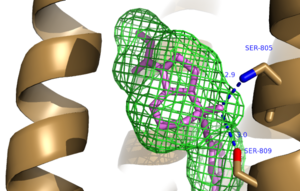
| - | A number of intramolecular interactions within the trans-membrane domain stabilize the inactive conformation of mGlu<sub>5</sub>, as demonstrated by <scene name='72/726409/Overview/5'>mGlu<sub>5</sub></scene> being represented in the inactivate state. While in the inactive state, glutamate binding to mGlu<sub>5</sub> triggers a conformational change that leads to mGlu<sub>5</sub> to be in the active state and hence triggers the aforementioned [https://en.wikipedia.org/wiki/Gq_alpha_subunit G<sub>q</sub> pathway]. The first of these interactions is an ionic interaction, termed the <scene name='72/726409/Ionic_lock2/2'>Ionic Lock</scene>, between Lysine 665 of TM3 and Glutamate 770 of TM6. Evidence for the importance of this interaction came through a kinetic study of mutant proteins where both residues were separately substituted with alanine, resulting in constitutive activity of the GPCR and its coupled pathway.<ref name="Primary">PMID: 25042998 </ref> A second critical interaction that stabilizes the inactive conformer is a <scene name='72/726409/Hydrogen_bond_614-668/2'>Hydrogen Bond </scene> between Serine 614 of ICL1 and Arginine 668 of TM3. Similarly, when Serine 614 was substituted with alanine, high levels of activity were seen in the mutant GPCR.<ref name="Primary">PMID: 25042998 </ref> A <scene name='72/726404/Scene_6/8'>Disulfide Bond </scene> between Cysteine 644 of TM3 and Cysteine 733 of <scene name='72/726409/Mavoglurant_overview2/ | + | A number of intramolecular interactions within the trans-membrane domain stabilize the inactive conformation of mGlu<sub>5</sub>, as demonstrated by <scene name='72/726409/Overview/5'>mGlu<sub>5</sub></scene> being represented in the inactivate state. While in the inactive state, glutamate binding to mGlu<sub>5</sub> triggers a conformational change that leads to mGlu<sub>5</sub> to be in the active state and hence triggers the aforementioned [https://en.wikipedia.org/wiki/Gq_alpha_subunit G<sub>q</sub> pathway]. The first of these interactions is an ionic interaction, termed the <scene name='72/726409/Ionic_lock2/2'>Ionic Lock</scene>, between Lysine 665 of TM3 and Glutamate 770 of TM6. Evidence for the importance of this interaction came through a kinetic study of mutant proteins where both residues were separately substituted with alanine, resulting in constitutive activity of the GPCR and its coupled pathway.<ref name="Primary">PMID: 25042998 </ref> A second critical interaction that stabilizes the inactive conformer is a <scene name='72/726409/Hydrogen_bond_614-668/2'>Hydrogen Bond </scene> between Serine 614 of ICL1 and Arginine 668 of TM3. Similarly, when Serine 614 was substituted with alanine, high levels of activity were seen in the mutant GPCR.<ref name="Primary">PMID: 25042998 </ref> A <scene name='72/726404/Scene_6/8'>Disulfide Bond </scene> between Cysteine 644 of TM3 and Cysteine 733 of <scene name='72/726409/Mavoglurant_overview2/5'>ECL2</scene> is critical at anchoring ECL2 and is highly conserved across Class C GPCR’s.<ref name="Primary">PMID: 25042998 </ref> The ECL2's presence combined with the helical bundle of the trans-membrane domain creates a <scene name='72/726409/Electrogradient2/9'>Binding Cap</scene> that restricts entrance to the allosteric binding site within the seven trans-membrane α-helices. This restricted entrance has no effect on the natural ligand, glutamate, as it binds to the extracellular domain, but this entrance dictates potential drug targets that act through allosteric modulation.<ref name="Primary">PMID: 25042998 </ref> |
== Clinical Relevance == | == Clinical Relevance == | ||
===Role in Diseases=== | ===Role in Diseases=== | ||
Revision as of 02:13, 17 April 2016
| |||||||||||
References
- ↑ Vassilatis DK, Hohmann JG, Zeng H, Li F, Ranchalis JE, Mortrud MT, Brown A, Rodriguez SS, Weller JR, Wright AC, Bergmann JE, Gaitanaris GA. The G protein-coupled receptor repertoires of human and mouse. Proc Natl Acad Sci U S A. 2003 Apr 15;100(8):4903-8. Epub 2003 Apr 4. PMID:12679517 doi:http://dx.doi.org/10.1073/pnas.0230374100
- ↑ 2.0 2.1 Venkatakrishnan AJ, Deupi X, Lebon G, Tate CG, Schertler GF, Babu MM. Molecular signatures of G-protein-coupled receptors. Nature. 2013 Feb 14;494(7436):185-94. doi: 10.1038/nature11896. PMID:23407534 doi:http://dx.doi.org/10.1038/nature11896
- ↑ Pin JP, Galvez T, Prezeau L. Evolution, structure, and activation mechanism of family 3/C G-protein-coupled receptors. Pharmacol Ther. 2003 Jun;98(3):325-54. PMID:12782243
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 4.9 Dore AS, Okrasa K, Patel JC, Serrano-Vega M, Bennett K, Cooke RM, Errey JC, Jazayeri A, Khan S, Tehan B, Weir M, Wiggin GR, Marshall FH. Structure of class C GPCR metabotropic glutamate receptor 5 transmembrane domain. Nature. 2014 Jul 31;511(7511):557-62. doi: 10.1038/nature13396. Epub 2014 Jul 6. PMID:25042998 doi:http://dx.doi.org/10.1038/nature13396
- ↑ Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci Lett. 1993 Nov 26;163(1):53-7. PMID:8295733
- ↑ 6.0 6.1 Li G, Jorgensen M, Campbell BM. Metabotropic glutamate receptor 5-negative allosteric modulators for the treatment of psychiatric and neurological disorders (2009-July 2013). Pharm Pat Anal. 2013 Nov;2(6):767-802. doi: 10.4155/ppa.13.58. PMID:24237242 doi:http://dx.doi.org/10.4155/ppa.13.58
- ↑ Fuxe K, Borroto-Escuela DO. Basimglurant for treatment of major depressive disorder: a novel negative allosteric modulator of metabotropic glutamate receptor 5. Expert Opin Investig Drugs. 2015;24(9):1247-60. doi:, 10.1517/13543784.2015.1074175. Epub 2015 Jul 29. PMID:26219727 doi:http://dx.doi.org/10.1517/13543784.2015.1074175
- ↑ Domin H, Szewczyk B, Wozniak M, Wawrzak-Wlecial A, Smialowska M. Antidepressant-like effect of the mGluR5 antagonist MTEP in an astroglial degeneration model of depression. Behav Brain Res. 2014 Oct 15;273:23-33. doi: 10.1016/j.bbr.2014.07.019. Epub 2014, Jul 18. PMID:25043733 doi:http://dx.doi.org/10.1016/j.bbr.2014.07.019