User:Kurt Corsbie/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 18: | Line 18: | ||
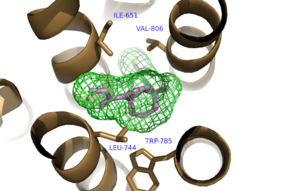
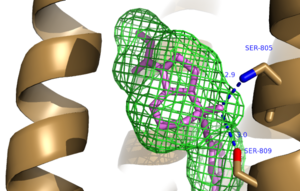
The bicyclic ring system of the drug is surrounded by a pocket of mainly hydrophobic residues including Val 806, Met 802, Phe 788, Trp 785, Leu 744, Ile 651, Pro 655, and Asn 747 (Figure 1).<ref name="Primary">PMID: 25042998 </ref> The carbamate tail of Mavoglurant forms a hydrogen bond through its carbonyl oxygen to the amide side-chain of Asparagine 747 of TM4 (Figure 2). A hydroxyl group similarly forms hydrogen bonds to mGlu<sub>5</sub>, specifically at two serine residues (S805 and S809) of TM7. These residues form a hydrogen bonding network to other residues through their main chain atoms and a coordinated water molecule (omitted for clarity) (Figure 3). The interactions between Mavoglurant and mGlu<sub>5</sub> involve TM helices that were not previously stabilized by any strong interactions, introducing a new level of stability that favors the inactive conformation of the protein and hence decreases the overall activity of mGlu<sub>5</sub>.<ref name="Primary">PMID: 25042998 </ref> | The bicyclic ring system of the drug is surrounded by a pocket of mainly hydrophobic residues including Val 806, Met 802, Phe 788, Trp 785, Leu 744, Ile 651, Pro 655, and Asn 747 (Figure 1).<ref name="Primary">PMID: 25042998 </ref> The carbamate tail of Mavoglurant forms a hydrogen bond through its carbonyl oxygen to the amide side-chain of Asparagine 747 of TM4 (Figure 2). A hydroxyl group similarly forms hydrogen bonds to mGlu<sub>5</sub>, specifically at two serine residues (S805 and S809) of TM7. These residues form a hydrogen bonding network to other residues through their main chain atoms and a coordinated water molecule (omitted for clarity) (Figure 3). The interactions between Mavoglurant and mGlu<sub>5</sub> involve TM helices that were not previously stabilized by any strong interactions, introducing a new level of stability that favors the inactive conformation of the protein and hence decreases the overall activity of mGlu<sub>5</sub>.<ref name="Primary">PMID: 25042998 </ref> | ||
| - | Mavoglurant was met by disappointing Fragile X Syndrome trial results and ultimately discontinued in 2014, but testing for dyskinesia and [https://en.wikipedia.org/wiki/Obsessive%E2%80%93compulsive_disorder Obsessive-compulsive disorder] are still in progress. [https://en.wikipedia.org/wiki/Basimglurant Basimglurant] and [https://en.wikipedia.org/wiki/MTEP MTEP] are inhibitors that are similar to Mavoglurant as they both function as negative allosteric modulators to mGlu<sub>5</sub>, and they are currently under trials to serve as medications for depression.<ref name="Clinical">PMID: 26219727 </ref><ref name=”Clinical2”>PMID: 25043733 </ref> | + | Mavoglurant was met by disappointing Fragile X Syndrome trial results and ultimately discontinued for Fragile X Syndrome in 2014, but testing for dyskinesia and [https://en.wikipedia.org/wiki/Obsessive%E2%80%93compulsive_disorder Obsessive-compulsive disorder] are still in progress. [https://en.wikipedia.org/wiki/Basimglurant Basimglurant] and [https://en.wikipedia.org/wiki/MTEP MTEP] are inhibitors that are similar to Mavoglurant as they both function as negative allosteric modulators to mGlu<sub>5</sub>, and they are currently under trials to serve as medications for depression.<ref name="Clinical">PMID: 26219727 </ref><ref name=”Clinical2”>PMID: 25043733 </ref> |
[[Image:Mav_Hydrophobic_pocket.png |300 px|left|thumb|Figure 1. Hydrophobic Pocket Surrounding Mavoglurant]] | [[Image:Mav_Hydrophobic_pocket.png |300 px|left|thumb|Figure 1. Hydrophobic Pocket Surrounding Mavoglurant]] | ||
Revision as of 02:15, 17 April 2016
| |||||||||||
References
- ↑ Vassilatis DK, Hohmann JG, Zeng H, Li F, Ranchalis JE, Mortrud MT, Brown A, Rodriguez SS, Weller JR, Wright AC, Bergmann JE, Gaitanaris GA. The G protein-coupled receptor repertoires of human and mouse. Proc Natl Acad Sci U S A. 2003 Apr 15;100(8):4903-8. Epub 2003 Apr 4. PMID:12679517 doi:http://dx.doi.org/10.1073/pnas.0230374100
- ↑ 2.0 2.1 Venkatakrishnan AJ, Deupi X, Lebon G, Tate CG, Schertler GF, Babu MM. Molecular signatures of G-protein-coupled receptors. Nature. 2013 Feb 14;494(7436):185-94. doi: 10.1038/nature11896. PMID:23407534 doi:http://dx.doi.org/10.1038/nature11896
- ↑ Pin JP, Galvez T, Prezeau L. Evolution, structure, and activation mechanism of family 3/C G-protein-coupled receptors. Pharmacol Ther. 2003 Jun;98(3):325-54. PMID:12782243
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 4.9 Dore AS, Okrasa K, Patel JC, Serrano-Vega M, Bennett K, Cooke RM, Errey JC, Jazayeri A, Khan S, Tehan B, Weir M, Wiggin GR, Marshall FH. Structure of class C GPCR metabotropic glutamate receptor 5 transmembrane domain. Nature. 2014 Jul 31;511(7511):557-62. doi: 10.1038/nature13396. Epub 2014 Jul 6. PMID:25042998 doi:http://dx.doi.org/10.1038/nature13396
- ↑ Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci Lett. 1993 Nov 26;163(1):53-7. PMID:8295733
- ↑ 6.0 6.1 Li G, Jorgensen M, Campbell BM. Metabotropic glutamate receptor 5-negative allosteric modulators for the treatment of psychiatric and neurological disorders (2009-July 2013). Pharm Pat Anal. 2013 Nov;2(6):767-802. doi: 10.4155/ppa.13.58. PMID:24237242 doi:http://dx.doi.org/10.4155/ppa.13.58
- ↑ Fuxe K, Borroto-Escuela DO. Basimglurant for treatment of major depressive disorder: a novel negative allosteric modulator of metabotropic glutamate receptor 5. Expert Opin Investig Drugs. 2015;24(9):1247-60. doi:, 10.1517/13543784.2015.1074175. Epub 2015 Jul 29. PMID:26219727 doi:http://dx.doi.org/10.1517/13543784.2015.1074175
- ↑ Domin H, Szewczyk B, Wozniak M, Wawrzak-Wlecial A, Smialowska M. Antidepressant-like effect of the mGluR5 antagonist MTEP in an astroglial degeneration model of depression. Behav Brain Res. 2014 Oct 15;273:23-33. doi: 10.1016/j.bbr.2014.07.019. Epub 2014, Jul 18. PMID:25043733 doi:http://dx.doi.org/10.1016/j.bbr.2014.07.019