We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1165
From Proteopedia
(Difference between revisions)
| Line 16: | Line 16: | ||
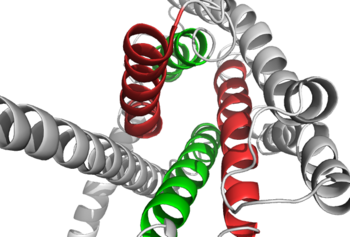
==How These Structures Lead to Function== | ==How These Structures Lead to Function== | ||
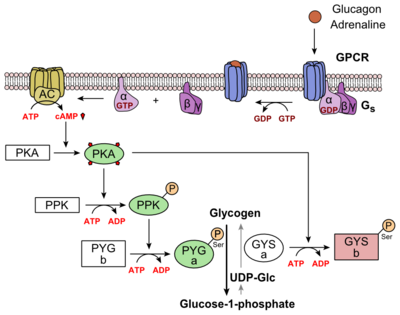
| - | Structurally, the 7TM and its signature seven helical structure is involved in [https://en.wikibooks.org/wiki/Principles_of_Biochemistry/Signaling_inside_the_Cell signaling] via [https://en.wikibooks.org/wiki/Structural_Biochemistry/Energy_coupling_in_chemical_reactions coupling] to [https://en.wikipedia.org/wiki/Heterotrimeric_G_protein heterotrimeric G proteins] that activate [https://en.wikipedia.org/wiki/Adenylyl_cyclase adenylate cyclase] to increase the levels of intracellular [https://en.wikipedia.org/wiki/Cyclic_adenosine_monophosphate cyclic AMP]. Additionally, this coupling increases levels of [https://en.wikipedia.org/wiki/Inositol_phosphate IP3] and intracellular [https://en.wikipedia.org/wiki/Calcium calcium] levels. <ref name="Tips">PMID: 23863937</ref> The wider and deeper ligand-binding pocket of class B GPCRs allows for a vast array of molecules to be bound that in turn allow for numerous functions activated by peptide [https://en.wikipedia.org/wiki/Receptor_(biochemistry) receptors]. <ref name="Ligands">PMID: 21542831</ref> The conformation and orientation of the 7TM and the ECD regions dictate the functionality of the class B G protein-coupled receptor, which has an open and closed [https://en.wikipedia.org/wiki/Conformation conformation] of the GCGR. When glucagon binds to GCGR, the open conformation of GCGR is stabilized. | + | Structurally, the 7TM and its signature seven helical structure is involved in [https://en.wikibooks.org/wiki/Principles_of_Biochemistry/Signaling_inside_the_Cell signaling] via [https://en.wikibooks.org/wiki/Structural_Biochemistry/Energy_coupling_in_chemical_reactions coupling] to [https://en.wikipedia.org/wiki/Heterotrimeric_G_protein heterotrimeric G proteins] that activate [https://en.wikipedia.org/wiki/Adenylyl_cyclase adenylate cyclase] to increase the levels of intracellular [https://en.wikipedia.org/wiki/Cyclic_adenosine_monophosphate cyclic AMP]. Additionally, this coupling increases levels of [https://en.wikipedia.org/wiki/Inositol_phosphate IP3] and intracellular [https://en.wikipedia.org/wiki/Calcium calcium] levels. <ref name="Tips">PMID: 23863937</ref> The wider and deeper ligand-binding pocket of class B GPCRs allows for a vast array of molecules to be bound that in turn allow for numerous functions activated by peptide [https://en.wikipedia.org/wiki/Receptor_(biochemistry) receptors]. <ref name="Ligands">PMID: 21542831</ref> The conformation and orientation of the 7TM and the ECD regions dictate the functionality of the class B G protein-coupled receptor, which has an open and closed [https://en.wikipedia.org/wiki/Conformation conformation] of the GCGR. When glucagon binds to GCGR, the open conformation of GCGR is stabilized. The open conformation is when glucagon can bind to GCGR; in the closed conformation binding does not occur.<ref name="Ligands">PMID: 21542831</ref> |
| Line 27: | Line 27: | ||
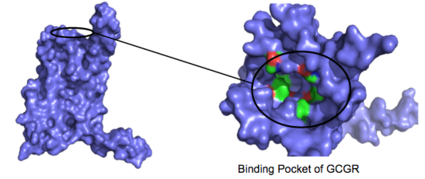
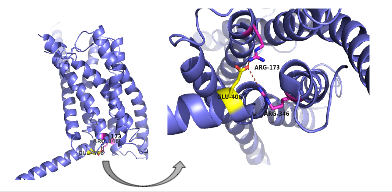
| - | There are specific amino acid interactions that hold the helices of the 7TM in the conformation that maximizes [http://www.chemicool.com/definition/affinity.html affinity]. <ref name="Ligands">PMID: 21542831</ref> This includes the [https://en.wikipedia.org/wiki/Disulfide disulfide bond] between <scene name='72/721535/Disulfide_bond_notspin/1'> Cys 294 and Cys 224</scene> that serves to hold the helices in the proper orientation for binding. Additionally, the [https://en.wikipedia.org/wiki/Salt_bridge_%28protein_and_supramolecular%29 salt bridges] between Glu 406, Arg 173, and Arg 346, also mentioned earlier, hold the conformation together for higher affinity (Figure 3). <ref name="Ligands">PMID: 21542831</ref> Finally, alpha helical structure of the stalk is | + | There are specific amino acid interactions that hold the helices of the 7TM in the conformation that maximizes [http://www.chemicool.com/definition/affinity.html affinity]. <ref name="Ligands">PMID: 21542831</ref> This includes the [https://en.wikipedia.org/wiki/Disulfide disulfide bond] between <scene name='72/721535/Disulfide_bond_notspin/1'> Cys 294 and Cys 224</scene> that serves to hold the helices in the proper orientation for binding. Additionally, the [https://en.wikipedia.org/wiki/Salt_bridge_%28protein_and_supramolecular%29 salt bridges] between Glu 406, Arg 173, and Arg 346, also mentioned earlier, hold the conformation together for higher affinity (Figure 3). <ref name="Ligands">PMID: 21542831</ref> Finally, alpha helical structure of the <scene name='72/721535/Opening_orientation/2'>stalk</scene> is required for high affinity conformation. The high affinity conformation of GCGR is the open conformation when glucagon can bind. Without these specific interactions between the residues open conformation is not stabilized and GCGR remains in the closed conformation where glucagon cannot bind. <ref name="Tips">PMID: 23863937</ref> |
Revision as of 23:20, 18 April 2016
References
- ↑ 1.0 1.1 Hollenstein K, de Graaf C, Bortolato A, Wang MW, Marshall FH, Stevens RC. Insights into the structure of class B GPCRs. Trends Pharmacol Sci. 2014 Jan;35(1):12-22. doi: 10.1016/j.tips.2013.11.001. Epub, 2013 Dec 18. PMID:24359917 doi:http://dx.doi.org/10.1016/j.tips.2013.11.001
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Siu FY, He M, de Graaf C, Han GW, Yang D, Zhang Z, Zhou C, Xu Q, Wacker D, Joseph JS, Liu W, Lau J, Cherezov V, Katritch V, Wang MW, Stevens RC. Structure of the human glucagon class B G-protein-coupled receptor. Nature. 2013 Jul 25;499(7459):444-9. doi: 10.1038/nature12393. Epub 2013 Jul 17. PMID:23863937 doi:10.1038/nature12393
- ↑ 3.0 3.1 3.2 3.3 3.4 Miller LJ, Dong M, Harikumar KG. Ligand binding and activation of the secretin receptor, a prototypic family B G protein-coupled receptor. Br J Pharmacol. 2012 May;166(1):18-26. doi: 10.1111/j.1476-5381.2011.01463.x. PMID:21542831 doi:http://dx.doi.org/10.1111/j.1476-5381.2011.01463.x
- ↑ Thomsen J, Kristiansen K, Brunfeldt K, Sundby F. The amino acid sequence of human glucagon. FEBS Lett. 1972 Apr 1;21(3):315-319. PMID:11946536
- ↑ 5.0 5.1 5.2 Bortolato A, Dore AS, Hollenstein K, Tehan BG, Mason JS, Marshall FH. Structure of Class B GPCRs: new horizons for drug discovery. Br J Pharmacol. 2014 Jul;171(13):3132-45. doi: 10.1111/bph.12689. PMID:24628305 doi:http://dx.doi.org/10.1111/bph.12689
- ↑ Mukund S, Shang Y, Clarke HJ, Madjidi A, Corn JE, Kates L, Kolumam G, Chiang V, Luis E, Murray J, Zhang Y, Hotzel I, Koth CM, Allan BB. Inhibitory mechanism of an allosteric antibody targeting the glucagon receptor. J Biol Chem. 2013 Nov 4. PMID:24189067 doi:http://dx.doi.org/10.1074/jbc.M113.496984
- ↑ Hoare SR. Allosteric modulators of class B G-protein-coupled receptors. Curr Neuropharmacol. 2007 Sep;5(3):168-79. doi: 10.2174/157015907781695928. PMID:19305799 doi:http://dx.doi.org/10.2174/157015907781695928
- ↑ 8.0 8.1 8.2 Yang L, Yang D, de Graaf C, Moeller A, West GM, Dharmarajan V, Wang C, Siu FY, Song G, Reedtz-Runge S, Pascal BD, Wu B, Potter CS, Zhou H, Griffin PR, Carragher B, Yang H, Wang MW, Stevens RC, Jiang H. Conformational states of the full-length glucagon receptor. Nat Commun. 2015 Jul 31;6:7859. doi: 10.1038/ncomms8859. PMID:26227798 doi:http://dx.doi.org/10.1038/ncomms8859