We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1160
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
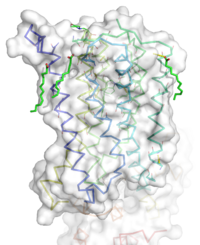
[[Image:STR.png|200 px|left|thumb|'''Figure 1''': Overall Structure of the mGlu5 TMD. The polar heads on the Oliec acids orient the image with the top oriented extracellularly, the middle portion inserted into the membrane, and the lower portion oriented intracellularly. The white exterior represents the surface of the protien, and the multicolored lines interior to the surface represent the backbones 7 transmembrane alpha helices. ]] | [[Image:STR.png|200 px|left|thumb|'''Figure 1''': Overall Structure of the mGlu5 TMD. The polar heads on the Oliec acids orient the image with the top oriented extracellularly, the middle portion inserted into the membrane, and the lower portion oriented intracellularly. The white exterior represents the surface of the protien, and the multicolored lines interior to the surface represent the backbones 7 transmembrane alpha helices. ]] | ||
=== Overview === | === Overview === | ||
| - | The mGlu5 TMD contains 7 <scene name='72/721531/Protien_7_helices/4'> alpha helices</scene> that spans the membrane. The protein was crystallized with <scene name='72/721531/Protien_clean_sce/6'>Oleic acid</scene> shown in <font color='red'>'''red'''</font>. On the top portion of the protein several critical extracellular loops connect the TMD to the CRD. The binding pocket can be found near the middle of the protein, and is mainly comprised of hydrophobic amino acids with more polar amino acids found in the upper and lower portions of the binding site. Inserted into the biding pocket is the negative allosteric modulator [http://www.en.wikipedia.org/wiki/Mavoglurant mavoglurant]. The TMD is in an inactive conformation, since mavoglurant is bound. Also, the deletion of the flexible domains leaves the mGlu5 receptor unable to bind to its [[GPCR]]. The inactive state is maintained by multiple ionic locks whose positions determine the active versus inactive conformation. | + | The mGlu5 TMD contains 7 <scene name='72/721531/Protien_7_helices/4'> alpha helices</scene> that spans the membrane. The protein was crystallized with <scene name='72/721531/Protien_clean_sce/6'>Oleic acid</scene> shown in <font color='red'>'''red'''</font>. On the top portion of the protein several critical extracellular loops connect the TMD to the CRD. The binding pocket can be found near the middle of the protein, and is mainly comprised of hydrophobic amino acids with more polar amino acids found in the upper and lower portions of the binding site. Inserted into the biding pocket is the negative allosteric modulator [http://www.en.wikipedia.org/wiki/Mavoglurant mavoglurant]. The TMD is in an inactive conformation, since mavoglurant is bound. Also, the deletion of the flexible domains leaves the mGlu5 receptor unable to bind to its [[GPCR]]. The inactive state is maintained by multiple ionic locks whose positions determine the active versus inactive conformation. |
| + | |||
| + | It all begins with glutamate binding to the Venus flytrap domain. The signal is transduced across the cystine-rich domain to the TMD<ref name="Niswender" />. Next, the dimerization of the TMD occurs. This activates the Gq/11 pathway, which activates phospholipase Cβ<ref name="Niswender" />. The active [http://www.proteopedia.org/wiki/index.php/2zkm phospholipase Cβ] hydrolyzes phosphotinositides and generates [https://pubchem.ncbi.nlm.nih.gov/compound/439456#section=Top inositol 1,4,5-trisphosphate] and [http://www.sivabio.50webs.com/ip3.htm diacyl-glycerol]<ref name="Woodcock" />. This results in calcium mobilization and activation of PKC<ref name="Niswender" />. | ||
=== Extracellular Domain === | === Extracellular Domain === | ||
These are the <scene name='72/721532/Ecl_trail_7/2'>Extracellular Loops</scene> with extracellular loops (ECLs) <font color='purple'><b>1</b></font>, <font color='purple'><b>2</b></font>, and <font color='purple'><b>3</b></font> highlighted in <font color='purple'><b>purple</b></font>. Additionally in the ECL domain, a <scene name='72/721531/Ecl_trail_1/5'>disulfide bond</scene> is attached to both Helix 3 and the amino acid chain between Helix 5 and the N-terminus. <font color='teal'><b>Helix 3</b></font> and <font color='red'><b>Helix 5</b></font> are colored in <font color='teal'><b>teal</b></font> and <font color='red'><b>red</b></font> respectively. <font color='blue'><b>N-terminus</b></font> is represented in <font color='blue'><b>blue</b></font>. The <span style="color:yellow;background-color:black;font-weight:bold;">disulfide bond</span> is highlighted in <span style="color:yellow;background-color:black;font-weight:bold;">yellow</span>, and it is conserved in all classes of glutamate receptor 5 transmembrane domains<ref name="Wu" /> ECLs and the Helices are also factors that dictate how mavolgarent fits in the binding pocket (citations. The position of these factors can change the size of the binding pocket through tightening or widening. | These are the <scene name='72/721532/Ecl_trail_7/2'>Extracellular Loops</scene> with extracellular loops (ECLs) <font color='purple'><b>1</b></font>, <font color='purple'><b>2</b></font>, and <font color='purple'><b>3</b></font> highlighted in <font color='purple'><b>purple</b></font>. Additionally in the ECL domain, a <scene name='72/721531/Ecl_trail_1/5'>disulfide bond</scene> is attached to both Helix 3 and the amino acid chain between Helix 5 and the N-terminus. <font color='teal'><b>Helix 3</b></font> and <font color='red'><b>Helix 5</b></font> are colored in <font color='teal'><b>teal</b></font> and <font color='red'><b>red</b></font> respectively. <font color='blue'><b>N-terminus</b></font> is represented in <font color='blue'><b>blue</b></font>. The <span style="color:yellow;background-color:black;font-weight:bold;">disulfide bond</span> is highlighted in <span style="color:yellow;background-color:black;font-weight:bold;">yellow</span>, and it is conserved in all classes of glutamate receptor 5 transmembrane domains<ref name="Wu" /> ECLs and the Helices are also factors that dictate how mavolgarent fits in the binding pocket (citations. The position of these factors can change the size of the binding pocket through tightening or widening. | ||
| Line 28: | Line 30: | ||
=== Ionic Locks === | === Ionic Locks === | ||
Another important structural feature is the series of <scene name='72/721531/Ionic_lock/5'>ionic locks</scene> on the intracellular side of the protein. Interactions between these five amino acids will form a salt bridge, which will stabilize the inactive conformation<ref name="Dore" />. The primary ionic lock forms between Glu770, Lys665, and Ser613<ref name="Dore" />. A secondary ionic lock occurs between Ser614 and Arg668<ref name="Dore" />. The purpose of these ionic locks is analogous to the ionic interactions that stabilize the T state in [[Hemoglobin]]. In the case of the TMD of mGlu5, the ionic lock is formed when the NAM mavoglurant is bound. This ionic lock stabilizes the inactive state, where the intracellular loops are stabilized facing inwards<ref name="Wu" />. This conformational change will effectively block the crevice that is involved in binding the G-protein<ref name="Wu" />. Models have suggested that, even in a glutamate bound state, the mavoglurant bound receptor would be dimerized but incapable of signaling<ref name="Wu" />. This signaling incapable mGlu5 dimer will help maintain the readiness of the pathway, while still decreasing signal response. | Another important structural feature is the series of <scene name='72/721531/Ionic_lock/5'>ionic locks</scene> on the intracellular side of the protein. Interactions between these five amino acids will form a salt bridge, which will stabilize the inactive conformation<ref name="Dore" />. The primary ionic lock forms between Glu770, Lys665, and Ser613<ref name="Dore" />. A secondary ionic lock occurs between Ser614 and Arg668<ref name="Dore" />. The purpose of these ionic locks is analogous to the ionic interactions that stabilize the T state in [[Hemoglobin]]. In the case of the TMD of mGlu5, the ionic lock is formed when the NAM mavoglurant is bound. This ionic lock stabilizes the inactive state, where the intracellular loops are stabilized facing inwards<ref name="Wu" />. This conformational change will effectively block the crevice that is involved in binding the G-protein<ref name="Wu" />. Models have suggested that, even in a glutamate bound state, the mavoglurant bound receptor would be dimerized but incapable of signaling<ref name="Wu" />. This signaling incapable mGlu5 dimer will help maintain the readiness of the pathway, while still decreasing signal response. | ||
| - | |||
| - | == Pathway == | ||
| - | It all begins with glutamate binding to the Venus flytrap domain. The signal is transduced across the cystine-rich domain to the TMD<ref name="Niswender" />. Next, the dimerization of the TMD occurs. This activates the Gq/11 pathway, which activates phospholipase Cβ<ref name="Niswender" />. The active [http://www.proteopedia.org/wiki/index.php/2zkm phospholipase Cβ] hydrolyzes phosphotinositides and generates [https://pubchem.ncbi.nlm.nih.gov/compound/439456#section=Top inositol 1,4,5-trisphosphate] and [http://www.sivabio.50webs.com/ip3.htm diacyl-glycerol]<ref name="Woodcock" />. This results in calcium mobilization and activation of PKC<ref name="Niswender" />. | ||
== Disease == | == Disease == | ||
Revision as of 01:39, 19 April 2016
Human metabotropic glutamate receptor 5 transmembrane domain
| |||||||||||